- 1Molecular Oncology and Angiogenesis Unit, IRCCS AOU San Martino-IST, Genoa, Italy
- 2Division of Immunology, Transplants and Infectious Diseases, Scientific Institute San Raffaele, Milan, Italy
Increasing evidences have pointed out the relevance of natural killer (NK) cells in organ-specific and systemic autoimmune diseases. NK cells bear a plethora of activating and inhibiting receptors that can play a role in regulating reactivity with autologous cells. The activating receptors recognize natural ligands up-regulated on virus-infected or stressed or neoplastic cells. Of note, several autoimmune diseases are thought to be linked to viral infections as one of the first event in inducing autoimmunity. Also, it is conceivable that autoimmunity can be triggered when a dysregulation of innate immunity occurs, activating T and B lymphocytes to react with self-components. This would imply that NK cells can play a regulatory role during adaptive immunity; indeed, innate lymphoid cells (ILCs), comprising the classical CD56+ NK cells, have a role in maintaining or alternating tissue homeostasis secreting protective and/or pro-inflammatory cytokines. In addition, NK cells display activating receptors involved in natural cytotoxicity and the activating isoforms of receptors for HLA class I that can interact with healthy host cells and induce damage without any evidence of viral infection or neoplastic-induced alteration. In this context, the interrelationship among ILC, extracellular-matrix components, and mesenchymal stromal cells can be considered a key point for the control of homeostasis. Herein, we summarize evidences for a role of NK cells in autoimmune diseases and will give a point of view of the interplay between NK cells and self-cells in triggering autoimmunity.
Introduction
Natural killer (NK) cells are one of the main components of innate immunity [reviewed in Ref. (1–7)]. It is thought that they provide the body with a strong defense against microorganisms, such as viruses and bacteria, together with their efficient action in limiting neoplastic cell growth (1). The functional definition of NK cells, that is their ability of killing other cells without any prior stimulation, implies that different cell populations can have the functional characteristics of NK cells without sharing a common phenotype. The large majority of the surface markers able to identify this cell population are actually expressed by other kinds of lymphocytes leading to an intrinsic difficulty in defining a cell as an NK cells on the basis of phenotype. As several other components of the innate arm of the immune system, NK cells can secrete cytokines and chemokines. Both activation of cytolytic machinery and secretion of regulating soluble factors are dependent on a wide number of surface and intracellular receptors that, interacting with the appropriate ligand, can lead to activation or inhibition of a given cell function. As always in a biological system, the balance between these opposite signals is responsible for the final outcome in the microenvironment; thus, NK cells can influence and regulate the activities of adaptive immune responses, including T cells [reviewed in Ref. (8)] and dendritic cells (DCs) (9, 10) through well identified surface receptors. Recent findings have pointed out that NK cells may play important roles in autoimmune disorders; indeed, a genetic correlation between NK cell expression of HLA-I receptors and autoimmune diseases has been shown. In addition, it appears that NK cells may play opposite roles with both regulatory and inducer activity in some autoimmune diseases (11–25).
Functional Balance between Activating and Inhibiting Signals in NK Cells
It is well known that the functional behavior of NK cells can be regulated by positive and negative signals. A detailed analysis of positive and negative NK cell receptors is reported elsewhere (6). Roughly, two main systems of molecular regulators are expressed on NK cells: the first one is represented by invariant NK cell receptors for HLA-I while the second one is composed of several receptors which do not bind HLA-I. The molecular and functional characteristics of NK cell receptors for HLA-I have been extensively analyzed (26–28): briefly, killer immunoglobulin-like inhibitory receptors (KIRs) and C-lectin-type-inhibitory receptors (CLIRs) can recognize either unique or several HLA-I alleles blocking NK cell function. Some members of these receptors can be expressed on NK cells also in an activating isoform that, in the extracellular portion, is apparently identical to the inhibiting one, indicating that the same HLA-I allele product can be positively recognized as well. Furthermore, in some instances, only the activating form of a member of KIR family has been identified, although it is not still defined unequivocally its corresponding HLA-I ligand. All these findings would render the scenario of NK cell receptors for HLA-I much more complicated than it was supposed in the late 90s (29, 30). Regarding the non-HLA-I receptors present on NK cells, some are of the activating type such as CD69, NKp30, NKp44, NKp46, NKG2D, and DNAM1 (31–33), while others are of the inhibiting type as LAIR1 (34). It should be noted that the peculiar behavior of the 2B4 receptor, which can deliver an activating signal when the signal transducer called SAP/SH2D1A is present in the cytoplasm; but in some instances it can deliver an inhibiting signal also in the presence of this transducer [reviewed in Ref. (35, 36)]. It is commonly thought that NK cells do not aggress self-cells because the balance between negative and positive signals is always in favor of the negative regulation: this balance is broken when self-cells do not express HLA-I (as during viral infections) or up-regulate natural ligands for activating receptors as it happens during tumor transformation (10, 37).
Evidence for the Recognition of Self-Cell by NK Cells
Like T lymphocytes, NK cells should not recognize autologous cells, unless autoreactivity is triggered, potentially leading to an autoimmune disease. Based on the original definition of NK cells (1), in principle a self-cell can be killed by NK cells without any previous stimulation. To avoid this damage, a self-cell is equipped with two major molecular mechanisms: (a) strong expression of HLA-I antigens able to deliver inhibiting signals to NK cells; (b) low levels or lack of expression of surface ligands essential for triggering NK cell activation [reviewed in Ref. (8, 37)]. In the latter context, also the down-regulation of ligands for co-receptors of NK cell activation can play a key role in avoiding self-aggression [reviewed in Ref. (4, 38)].
It is becoming evident that NK cells can recognize self-cells, which express ligands for activating receptors (8); indeed, NK cells can aggress both T and antigen presenting cells (APCs) upon triggering with toll-like receptor (TLR) or stimulation with IL2 or IL15 cytokines. These stimuli lead to the up-regulation of NKG2D receptor or to the neo-expression of CD69 and NKp44, which in turn can trigger cytolytic activity and cytokine production (31, 39). On the other hand, several stimuli conceivably acting through the T cell receptor/CD3 complex, such as phytohemoagglutinin (PHA), alloantigens, superantigens, and antigenic peptides, can induce the neo-expression of NKG2D ligands (NKG2DL) on CD4+ and CD8+ T lymphocytes [reviewed in Ref. (8)]. Moreover, also microorganisms as HIV or Mycobacterium tuberculosis can trigger NKG2DL expression on CD4+ T cells and T regulatory (Treg) cells (40, 41). The NKG2DL are represented by stress-induced MHC class I-related molecules, such as MICA/B, or the UL16 binding proteins (ULBPs), that are indeed recognized not only by NK cells but also by a large number of “unconventional” T lymphocytes, as γδ T and NKT cells (11, 12, 42–44). It is conceivable that even CD8+ memory T cells could be triggered through NKG2DL; all these cell populations can lead, acting alone or together, to autoreactivity (11). Indeed, the duty of innate immunity is to clear the body from a specific pathogen or impede the development of cancer; thus, one can consider autoimmunity as a drawback of a defective lymphoid stress surveillance that does not limit properly the dissemination of infected or malignant cells and does not maintain tissue integrity, leading to an altered adaptive immune response. In addition, also the poliovirus receptor (PVR) or nectin-2, both ligands for DNAM1 (45) can be expressed on activated or HIV-infected CD4+ T cells possibly leading to NK cell recognition through the DNAM1 activating receptor. To our knowledge, no reports are present so far in the literature on the possible interactions between activated T cells and NK cell receptors, such as natural cytotoxicity receptors and/or 2B4, although the 2B4 ligand CD48 can be expressed on T, B, and NK cells [reviewed in Ref. (46)]. It has been shown in a mouse model that blocking of 2B4 with a 2B4-fusion protein inhibits the generation of autoimmune hepatitis (AIH) suggesting that a still undefined 2B4+ lymphocyte subset can be involved (47). This deserves further studies in humans to better clarify the molecular mechanisms of NK cell-T lymphocyte cross-talk. Nevertheless, these findings strongly indicate that NK cells can strikingly regulate T cell responses influencing adaptive immunity. In the adaptive immune response, APCs take a key role; indeed, APC can adequately expose the peptide antigen to allow its recognition by T cells (48). Different kinds of APC, with a reported different capacity of presenting the peptide antigen, can be identified (49–51). Focusing our analysis on monocyte and monocyte-derived dendritic cells (moDCs), it is known that NK cells can actively interact with these APC that produce interleukin 12 (also known as NK stimulating factor), which triggers both proliferation and cytolytic activity of NK cells (52). In turn, NK cells can produce cytokines, as TNFα, which contribute to DC cell maturation. Several reports have shown that IL2-activated NK cells can lyse self-APC and that NK–APC interaction may lead to cytokine production (9, 10, 49, 53, 54). Importantly, this interaction can be mediated by different activating receptors, including some natural cytotoxicity receptors, and by NKG2D or DNAM1 (9, 54–59). In addition, ligands for NKG2D can be up-regulated on APC upon stimulation with TLR-ligands, further supporting the idea that microbial infections can evoke an autoreactive response that leads to a limited adaptive immune response. Indeed, the NK cell-mediated elimination of a given APC before antigen presentation to T cells should conceivably impede an optimal T cell activation [reviewed in Ref. (10, 49)]; thus, also the second player of the adaptive immune response can be shut down by NK cells. Finally, on epithelial and mesodermal-derived cells, as well as on leukocytes, adhesive ligands such as the intercellular adhesion molecule-1 (ICAM1) can be up-regulated upon triggering by TLR or inflammatory cytokines, including IFNγ and TNFα (60). The counter receptor of ICAM1 is the lymphocyte function associated antigen-1 (LFA1), which is a major player of leukocyte-to-cell adhesion and NK cell activation [reviewed in Ref. (60–64)]. Of course, stress signals can up-regulate the ligands for NK cell activating receptors also on this cell population, favoring the NK cell-mediated self-aggression [reviewed in Ref. (37)]. These findings strongly suggest that the interaction between NK and self-cells during infection and/or inflammation should be the rule and not the exception; in addition, NK cells together with the so-called T cells with NK activity (primarily NKT and γδT cells) can down-regulate or even impede the generation of an adaptive immune response (43, 65, 66). It is conceivable that this interaction does not happen in the peripheral blood but within tissues or in the lymph nodes, at least in the case of organ-specific autoimmune diseases. In this context, several evidences have been reported on the presence of NK, NKT, or γδT lymphocytes, expressing NKG2D and DNAM1, among tissue infiltrating cells during autoimmune diseases; in the same tissues NKG2D and/or DNAM1 ligands are detectable. Indeed, these cells have been found in psoriatic, blistering diseases, and alopecia areata (AA) skin lesions (16, 22, 67–71), central nervous system (CNS) in multiple sclerosis (MS) patients (23, 25, 72–78) and synovial fluid in rheumatoid arthritis (RA) (17, 79–83).
Mesenchymal Stromal Cells as a Target for NK Cells
Within tissues NK cells can interact with other cells of innate immunity as monocyte-derived macrophages and dendritic cells, mesodermal cells, and extracellular-matrix components (EMCs) besides NKT and γδT cells (Figure 1). In particular, mesenchymal stromal cells (MSCs) are fibroblast-like cells responsible for the production of several extracellular-matrix proteins as collagen, vitronectin, fibronectin, and laminin, through which parenchymal cells can maintain both shape and functional interactions in a given organ. Among MSC, mesenchymal stem cells can undergo differentiation to stromal cells typical of connective tissues, including osteocytes, adipocytes, and chondrocytes [reviewed in Ref. (84)]. According to some experimental findings, the property to differentiate is not limited to cells of mesodermal origin but is also shared by ectodermal cells as neurons. Although conflicting results are reported in the literature, it is becoming evident that MSC can be a source of pluripotent stem cells that can be employed in tissue repair and regeneration. In addition, a functional common feature of MSC is the ability of regulating immune responses [Ref. (85); reviewed in Ref. (84, 86)]. Indeed, it has been shown that MSC derived from different tissues can down-regulate the activation of the immune system both in vitro and in vivo murine models. More importantly, these cells have been proposed as an additional therapeutic tool to control graft versus host disease (GVHD) in particular in children (87, 88). MSC can have a role in regulating autoreactivity through the modulation of cell-to-cell interactions and the production of extracellular-matrix proteins, cytokines, and enzymes [Ref. (85); reviewed in Ref. (84)]. The prevailing point of view of the literature is that MSC have a regulatory inhibiting role on several T and NK cell-mediated activities (87, 89). This regulation is reported to be mediated by soluble factors, such as TGFβ, HGF, IDO, and PGE2, which affect lymphocyte functions upon lymphocyte–MSC interaction [Ref. (85); reviewed in Ref. (84, 86, 89)]. On the other hand, it appears that NK and T cells can aggress MSC recognizing NKG2D and DNAM1 ligands, leading to MSC killing and release of pro-inflammatory cytokines (57, 90–92). This property is mainly confined to cytokine-activated NK cells, as ex vivo peripheral blood isolated NK cells are not efficient in MSC killing (90–93). It is of note that the regulatory role of MSC on NK cell functions is found in vitro at well defined MSC:NK cell ratios, ranging from 1:1 to 1:4, while at lower MSC–NK cell ratios the inhibiting effect is barely or not detectable and an activating effect is found (90). Due to in vitro culture conditions, at this ratios MSC grow as a monolayer covering the culture well, with lymphocytes seeded on them; thus, both extracellular-matrix proteins, as collagen and fibronectin, and inhibiting cytokines, as TGFβ, can be concentrated to the MSC surface and in the extracellular medium facilitating the delivery of an inhibiting signal to lymphocytes. On the other hand, it is still to be determined what happens during the interaction of a single MSC and an NK cell: it is conceivable that within connective tissues MSC–NK interactions take place in the presence of several extracellular-matrix proteins whose receptors are expressed on NK cells.
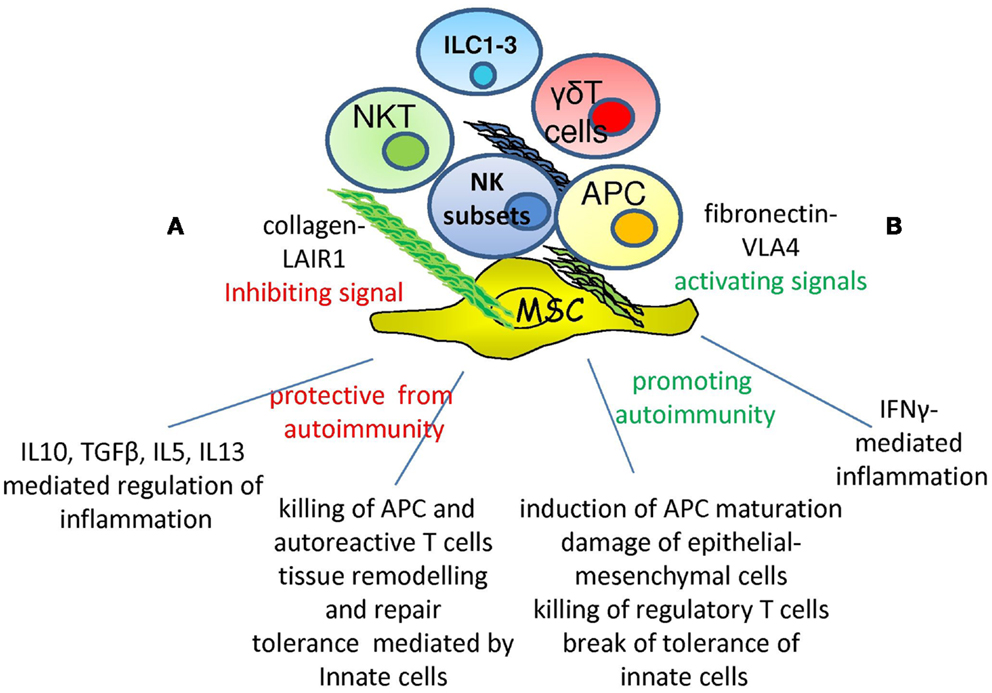
Figure 1. Opposite roles of NK cells in autoimmunity. Within microenvironment the interaction of specific NK cell receptor with extracellular-matrix can deliver different signals depending on the type of receptor involved: LAIR1–collagen interaction would lead to inhibition while VLA4-fibronectin engagement to activation. Depending on the type of NK cell subset involved, NK cells show the ability of protecting from the occurrence of autoimmunity (A) through the secretion of immune-regulating cytokines as IL10, TGFβ, IL5, and IL13. In addition, they can eliminate APC and autoreactive T cells through the triggering of activating receptors or regulate tissue homeostasis. On the other hand, NK cells can aggress tissues inducing inflammation through IFNγ production (B), favoring the maturation of APC with the consequent triggering of adaptive immune response. Killing and damaging parenchymal, epithelial, and mesenchymal cells eventually lead to an altered tissue homeostasis and then to autoimmunity. ILC1-3, NKT, and γδT cells are involved and may regulate the NK cell–microenvironment interactions.
Extracellular-Matrix Protein Receptors on NK Cells as Regulators of NK Cell Functions: Focus on the Leukocyte Associated Ig-Like Receptor 1
Natural killer cells can express different extracellular-matrix protein receptors as well as matrix metalloproteinases responsible for matrix degradation (94, 95). Some of these receptors are called very late antigen (VLA) as they are expressed on long-term cultured cells (Figure 1). However, some are constitutively expressed at the NK cell surface, such as VLA4 (96–98), and can also be up-regulated upon stimulation. Several different effects of NK cell interaction with the matrix proteins fibronectin, laminin, vitronectin, osteopontin, and collagen are reported in the literature (99–105) and their deep analysis is beyond the scope of this review. As an example, the engagement of VLA4 can induce activation of NK cells (98, 106–109). Herein, we focus on the leukocyte associated Ig-like receptor 1, LAIR1 or CD305 (110, 111), that has been shown to be a receptor for the Gly-Prol-Hyp common motif of collagens type I, II, III, XIII, XVII, and XXIII (112–115) (Figure 1). Importantly, LAIR1 is able to deliver an inhibiting signal which down-regulates NK cell activation through the CD16 receptor, reducing calcium mobilization, and the cytolytic activity triggered through this molecule (110, 116, 117). The LAIR1-mediated inhibiting signal occurs through the recruitment, by its cytoplasmic tail equipped with immunoreceptor tyrosine inhibiting motif (ITIM), of the SHIP1 phosphatase; this, in turn, impedes the phosphorylation and consequent activation of the immunoreceptor tyrosine activating motif (ITAM) present in the intracellular domain of several activating NK cell receptors (2, 118). LAIR1 can be expressed as different isoforms (LAIR1a, b, and c) or as a soluble form termed LAIR2; it is conceivable that the interaction of NK cells with collagens delivers a negative signal that may be impaired in the presence of soluble (s) LAIR (119–121). No direct evidence for the interaction of LAIR1 expressed by NK cells and collagen is reported so far; however, that indeed cross-linking of collagen can trigger an inhibiting signal in lymphocytes upon LAIR1 engagement has been demonstrated for T and B cells (117, 122–124), APCs (125, 126), and tumor cells (127–130). Altogether, these findings suggest that collagen produced by MSCs may be involved in the negative regulation of NK cell function. It is still to be defined which stimuli can regulate LAIR1 expression on NK cells. It is of note that LAIR1 is present on almost all leukocytes and it appears to be associated with the leukocyte common antigen (LCA) tyrosine phosphatase (CD45) on NK cells (131); thus, LAIR1 could regulate NK cell activation by itself and/or through the association with CD45. Interestingly, the lack or lower expression of LAIR1 is associated with an impaired inhibiting signal delivered upon LAIR1 engagement in B cells isolated from systemic lupus erythematosus (SLE) patients or B cell chronic leukemia (129, 132) supporting the idea that down-regulation of LAIR1 expression can be associated with autoimmune or neoplastic diseases.
NK Cell Subsets and Innate Lymphoid Cells as Players and Regulators of Autoimmunity
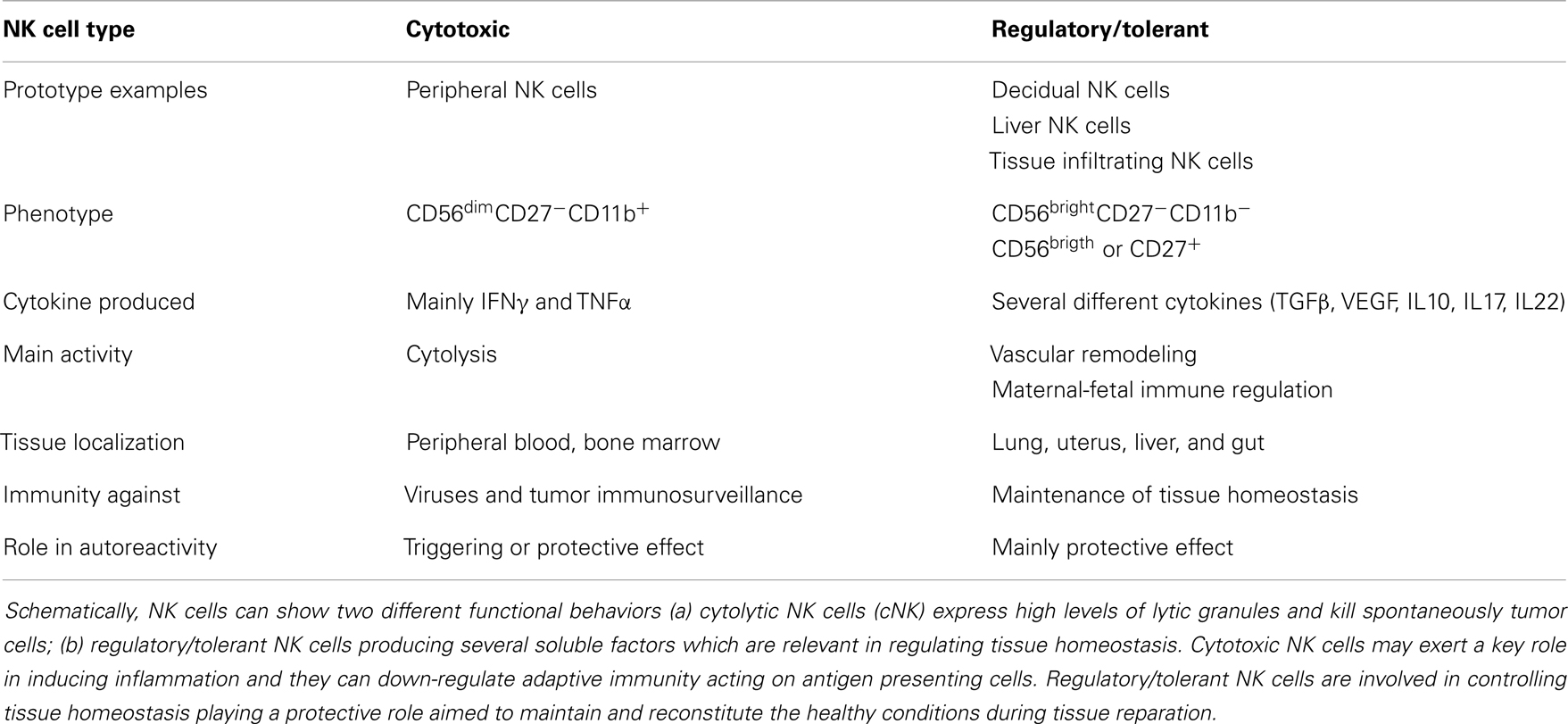
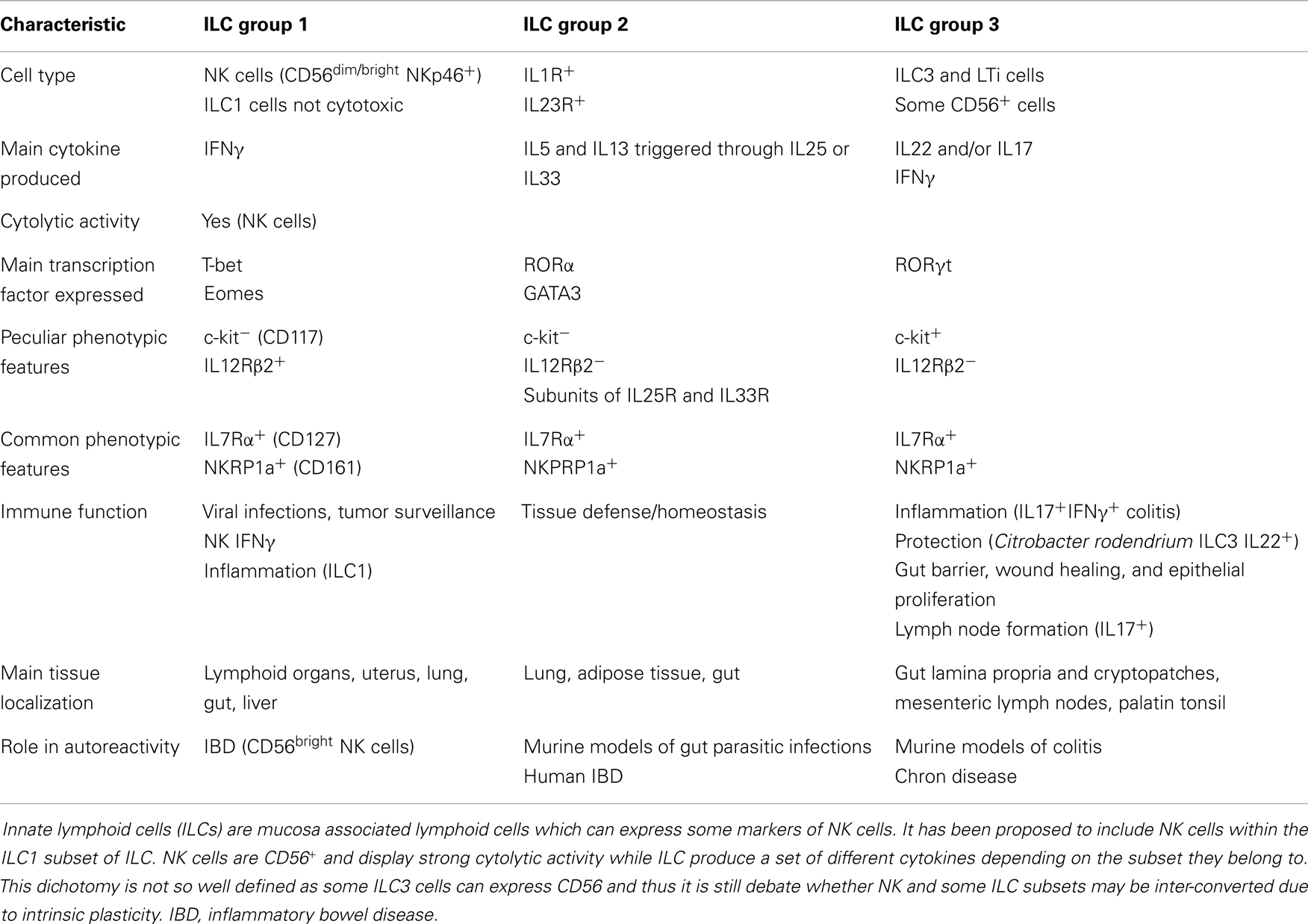
It is generally thought that autoreactivity and autoimmune diseases are based on an altered adaptive immune response determining the generation of T and B cell-mediated aggression of self-cells (133–136). This can be the result of a too strong reaction to self-antigen due to altered central or peripheral tolerance of autoreactive T and B cell clones. Treg cells are the main effectors of tolerance and several evidences have demonstrated that the lack of an optimal regulation of the adaptive immune response may be a consequence of their impaired function (137). NK cells can influence tolerance by eliminating Treg cells (15, 138, 139) or by acting as regulatory cells themselves (14, 21, 140–144). Indeed, upon engagement of activating receptors, NK cells can release several regulating cytokines, such as TGFβ and IL10, which are considered mediators of tolerance for T cells (5, 145). For instance, during viral infections, it is conceivable that the interaction of NK cells with infected self-cells results in the secretion of TGFβ and IL10, which in turn modulate T and B cell responses; of note, TGFβ is a strong down-regulator of NK cell-mediated activation and proliferation (146–149). Interestingly, secretion of functional TGFβ can be elicited in NK cells upon triggering with soluble HLA-I molecules that interact with the corresponding counter-receptors, as CD8 and/or the activating isoforms of KIRs and/or CLIRs (150, 151). An increment of sHLA-I can be detected in the sera of patients suffering from different autoimmune diseases; thus, one could suggest that sHLA-I can down-regulate NK cell activation. In addition, together with TGFβ, NK cells can release FasL (152); in turn, soluble FasL, interacting with Fas at the surface of lymphocytes, can lead to their cell death. Thus, the NK cell-mediated down-regulation of immune response may occur both by blocking activation with TGFβ and triggering cell death via FasL–Fas interaction (152). Recently, several distinct NK cell subsets have been found in different tissues playing opposite functional roles in immune response (Table 1). Briefly, it is commonly accepted that CD56dull and CD56bright NK cells present in the peripheral blood have distinct phenotype and functional activities. Indeed, CD16+KIR+CD56dull NK cells are primarily cytotoxic while the CD16−KIRdull CD56bright produce huge amounts of cytokines. It is not clear whether CD56dull posses the plasticity to become CD56bright and viceversa. Also, human NK cells can be subdivided on the basis of CD27 and CD11b expression (153, 154): the minority of peripheral NK cells is CD27+ (about 5%), while this population is more represented in the bone marrow and further in the spleen and tonsils. CD27+ NK cells, either CD11b+ or CD11b−, can produce high amounts of cytokines while among the CD27− NK cells those expressing CD11b are highly cytotoxic (Table 1). Of note, early during pregnancy the majority of human decidual lymphocytes are characterized by unique phenotype: CD16−CD11b−CD56bright either expressing or not CD27, CD9, and CD151 tetraspanning family members. Some of these cells can produce IL22 and express immunomodulatory molecules as galectin-1 and progestagen-associated protein 14 (155). Importantly, decidual NK (dNK) CD56brightCD27+ cells suppress Th17 through an IFNγ-dependent pathway and this population is lost in women with spontaneous abortion. Additional NK cell subsets, as NK2, NK3, NKr, and NK22 specifically involved in the secretion of immune-regulatory cytokines have been recently identified [reviewed in Ref. (21, 156–160)]. Subsets with a protective role in autoimmunity are NK2 cells, predominant in allergic disease, producing high amounts of IL4, IL5, and IL13 (161), NK3 cells which release IL10 (162), together with secreting TGFβ NKr cells which are involved in maternal-fetal immune tolerance (80) while NK22 cells limit inflammation and protect gut mucosal integrity through the action of IL22. To further complicate this scenario, innate lymphoid cells (ILCs, Table 2) distinct from NK cells, has been identified in mucosa associated lymphoid tissue (163). To uniform this variegate picture, it has been suggested to include NK cells within the ILC1 subset and it has been proposed that the CD56 molecule can be considered the best marker to distinguishing between NK and other lineage negative lymphoid cells (Table 2) as both kind of cells can express NKp46 and NKp44 receptors. More importantly, ILC1, ILC2, and ILC3 subsets express peculiar transcription factors as T-bet or RORα or RORγT (Table 2) resembling Th1, Th2, or Th17 T cell subsets respectively. Of note, ILC1, ILC2, and ILC3 cells are present in the gut and display a pro-inflammatory or a protective role depending on the main cytokine produced (Table 2). Finally, the NKp46−NKp44+RORγT+CD127+ NK cells show a protective role in autoimmunity but they may be counteracted by NKp46+NKp44−RORγT−CD127− NK cells which appear to be pathogenic through the production of IFNγ (164). Altogether these findings suggest that both different NK cell subsets and ILC are primarily involved in either host defense against viruses and tumor immunosurveillance or in regulating tissue homeostasis and autoimmunity. Furthermore, it is still to be determined the “plasticity” of an NK cell or ILC subset as it has been demonstrated for some T cell subsets [reviewed in Ref. (165, 166)].
NK Cells and Genetic Correlation with Autoimmune Diseases
Natural killer cell development and function is strictly related to genetic elements: the genetic background, particularly the defects and variations of KIR/HLA genotypes, can influence the function of a given NK cell receptor in target cell recognition and impair NK cell activation as well as self-tolerance. This influence is supposed to be related to autoimmunity (167); indeed, several findings have pointed out associations between risk of systemic or organ-specific autoimmune diseases and KIR/HLA genotypes, which indicate that self-tolerance may be broken with inappropriate receptor and ligand pairs or with the interrupted signal balance (38, 168–177). In general, the presence of an activating receptor for HLA-I associated with the lack or reduction of inhibitory pairs has been shown in several autoimmune diseases [reviewed in Ref. (21, 178)] suggesting that an imbalance in favor of activating receptors for HLA-I is associated with autoimmunity (Figure 2). This is in line with the findings observed in bone marrow transplantation where the expression of activating KIRs can override the regulating signals generated through inhibiting KIRs and/or CLIRs (179–182).
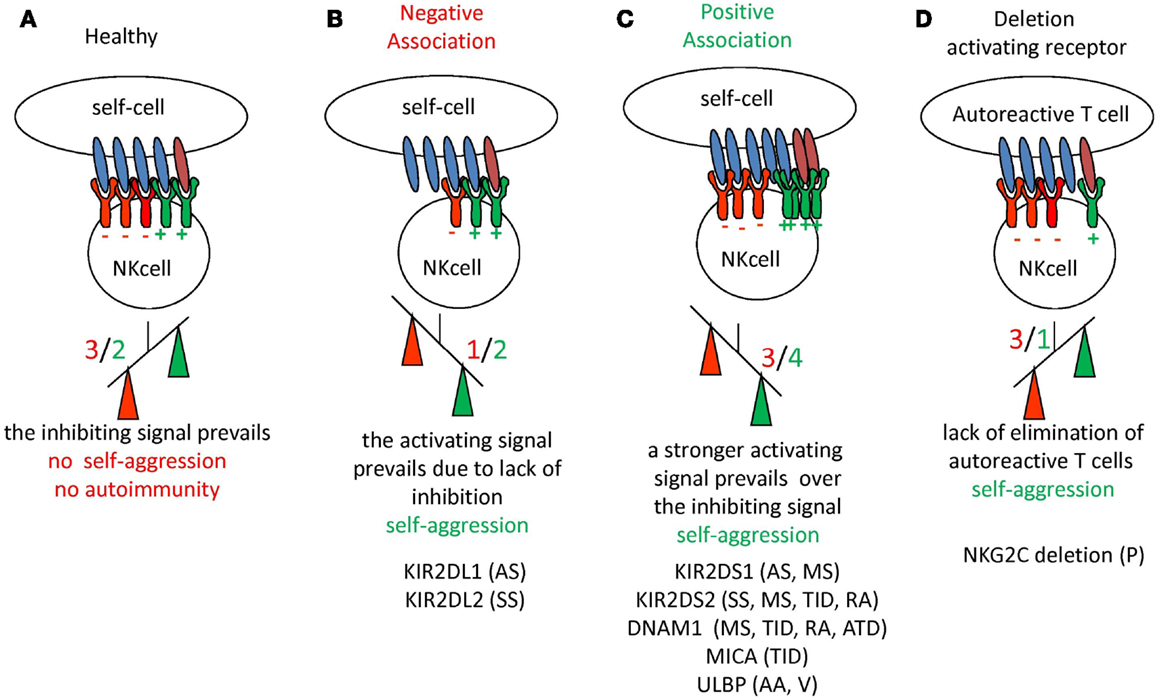
Figure 2. Schematic representation of the imbalance between inhibiting and activating receptors on NK cells with the occurrence of autoimmunity and autoimmune disease. (A) In healthy conditions the negative signal (in red) delivered through inhibiting receptors for HLA-I can prevail on the positive signal (in green) induced by the engagement of activating receptor. The net effect is that no damage to self-cells is induced, thus there is no autoreactivity. (B,C) The down-regulation of expression of inhibiting receptors (B) or an increment of activating receptors (C) determines in any case the prevalence of the activating signal on the inhibiting one, leading to self-reactivity (indicated as prevalence of positive signal shown in green). Although not shown, these two situations may also be found together. (D) In this case the lack of activating receptors can lead to impaired blocking of autoreactive T cell clones favoring autoreactivity. For each situation are listed the autoimmune diseases where an alteration of NK receptors have been reported. KIR2DS1 and KIR2DS2 are the activating isoforms of the NK receptor for HLA-C alleles. The KIR2DL1 and KIR2DL2 are the inhibiting isoforms of NK receptors for HLA-C alleles. NKG2C is the activating isoform of CLIR. DNAM1 is an activating receptor present on NK cells and MICA and ULBP are the ligands of the NKG2D surface receptor involved in the recognition of either infected or tumor transformed cells. AA, Alopecia areata; AS, ankylosing spondylitis; ATD, autoimmune thyroid disease; MS, multiple sclerosis; P, psoriasis; PV, pemphigus vulgaris; RA, rheumatoid arthritis; SS, systemic sclerosis; TID, type I diabetes; V, vasculitis.
In the following paragraphs, the findings regarding the potential role of NK cells in different autoimmune diseases will be listed and discussed.
Features of NK Cells in Skin Diseases
Typical autoimmune diseases of the skin are psoriasis, pemphigus vulgaris (PV), and AA; herein, we will not deal with skin specific manifestations of SLE which can be considered as a systemic autoimmune disorder with involvement of the skin. It has been reported that NK cells represent about 5–8% of infiltrating lymphocyte in psoriatic lesions and these cells are CD56brightCXCR3+CCR5+ cells (67) expressing the activation antigen CD69. These cells produce IFNγ after IL2 stimulation; in turn IFNγ can upregulate the HLA-I antigens on cheratinocytes and trigger activation of these cells as well. These findings would suggest that CD56+ NK cells can favor the development of psoriasis inducing local inflammation and amplify T cell autoimmune reactivity. This notion is further supported by the finding that CD56brightCXCR3+CCR5+ NK cells from psoriatic lesions can trigger cheratinocytes to produce CCL5 and CXCL10 chemokines which in turn favor NK cell chemotaxis. Of note, NK cells can also release IL22, a cytokine mainly produced by Th1 and Th17 T cells (183–185). Cheratinocytes incubated with IL22 can proliferate upon interaction with IL22 receptor (186) and this leads to parakeratosis and acanthosis, typical features of psoriatic lesions (187). Furthermore, genomic deletion of the activating receptor NKG2C is significantly increased in psoriatic patients compared to healthy matched controls (70, 188, 189). Thus, the lack of recognition by NK cells of autoreactive T cells may lead to exacerbation of psoriasis (Figure 2). In this context, the finding that NK cells bearing the inhibiting NK receptor NKG2A are incremented in skin psoriatic lesions can suggest that the imbalance between NKG2C+ and NKG2A+ NK cells may favor the expansion of autoreactive T cells (70). In AA, it has been found that CD56+NKG2D+ NK cells are mainly localized around and within the anagen hair follicles in prominent aggregates possibly leading to aggression of hair follicles favoring the collapse of the relative immune privilege of this cutaneous region (69). Finally, in PV it has been reported that peripheral NK cells display a Th2 type-biased phenotype (190) as they express high mRNA for IL10, a decrement of IL12Rβ, and produce IL5 in vivo, exclusively in patients with active disease compared to healthy control. Furthermore, NK cells may function as APCs for desmoglein three antigens to CD4+ T cells, suggesting also the possibility of a role for NK cells in inducing the tissue damage associated to PV (191).
NK Cells in Multiple Sclerosis
Multiple sclerosis is a CNS inflammatory autoimmune disease involving as target the myelin associated with neuronal axons; MS eventually leads to a progressive disability and host death due to the impairment of vital CNS functions. A potential pathogenic role of NK cells in MS is supported mainly in relapsing remitting MS patients (RRMS) [reviewed by Chanvillard et al. (23)]; indeed, NK cells can directly aggress and damage oligodendrocytes which produce myelin and NK cells are increased in MS lesions (192, 193). On the other hand, NK cells can directly affect the life of autoreactive T cells or APCs; in MS patients treated with IFNβ (194) or with the anti-CD25 antibody daclizumab, there is a selective expansion and activation of CD56bright NK cells and this correlates with a down-regulation of T cells activation and inhibition of inflammation (195, 196), suggesting that CD56bright NK cells are relevant in the control of MS lesions. Importantly, this NK cell subset appeared to kill T cells through granzyme K and A, which activate the mitochondrial pathway of apoptosis. The expansion of CD56bright NK cells can be dependent on their relative higher expression (compared to CD56dull NK cells) of the intermediate affinity receptor for IL2. Thus, during MS therapy CD56dull NK cells should be shut down through the blocking of the CD25 receptor by daclizumab; on the other hand, the expansion of CD56bright NK cells is favored because daclizumab does not impair their proliferation. Furthermore, in MS the NK2 cell subset is responsible for the production of IL5 and IL13, which may actively suppress the activity of self-reactive T cells. These cells disappear in MS patients when an exacerbation of the disease is present, while they re-appeared during the remission phase, suggesting that NK2 cells may be relevant for the disease control (197, 198).
NK Cells and Type I Diabetes
A reduction of peripheral NK cells has been reported in early diagnosed type I diabetes (TID) while the amount of NK cells is mostly similar to healthy controls in long-standing TID patients; more importantly, long-standing TID display lower amounts of IFNγ and lower expression of some natural cytotoxicity receptors (199) associated with high levels of glycosylated hemoglobin, suggesting that the impairment of NK cells could be a consequence of the disease. It is of note that some NK cells have been identified also within the pancreas, nearby to β pancreatic islets (200), although this finding has not been confirmed (201). In a murine diabetes model, it has been reported that NK cell are essential in abolishing the onset of the disease in NOD mice through a TGFβ-dependent mechanism that interferes with the activation of β-islet specific T cells (202, 203).
NK Cells in Rheumatoid Arthritis
It has been reported that NK cells producing IL22 and TNFα are increased in the synovial fluid of RA patients. It is of note that culture supernatants from these IL22-producing NK cells can trigger the proliferation of synovial fibroblast-like synoviocytes and this proliferation is inhibited using anti-IL22 and anti-TNFα antibodies (79). In addition, NK cells from synovial fluid are mainly CD56bright, express high levels of activation antigens and produce IFNγ. Furthermore, they can induce monocyte differentiation to dendritic cells, which in turn can trigger NK cells (204). Altogether these findings would suggest an active role of NK cells in sustaining inflammation in RA patients.
NK Cells in Inflammatory Bowel Disease
Inflammatory bowel diseases are represented by ulcerative colitis (UC) and Chron disease (CD): these two illnesses are characterized by the inflammation of gut accompanied by diarrhea and impairment of absorption of nutrients. It is commonly accepted that IL17A-producing lymphocytes are extremely relevant in IBD (205); among the different cell populations residing and colonizing (Th17, Th1-Th17, NKT, γδT cells) bowel mucosa in UC or CD, NK cells, and the group 3 of ILC3 (see Table 2) are good producer of IL17A. These cells can release IL17A immediately upon engagement with pathogen associated molecular patterns (PAMPs) and/or cytokines as IL23 (163). More importantly, both NK cells and ILC3 producing IFNγ and IL17 are abundant in inflamed CD mucosa while it is debated whether ILC3 secreting IL22 cells are increased or decreased in IBD (164, 206). It appears that some ILC of the subgroup 3 (Table 2) are relevant in the generation of the gut-associated lymphoid tissues and the maintenance of healthy conditions. In this context, the fine tuning of the respective functional role of colitogenic ILC producing IFNγ (ILC1 and some ILC3) and protective ILC3 secreting IL22 should be relevant in the generation of IBD.
NK Cells in Autoimmune Liver Diseases
Natural killer cells present in the healthy liver are different from those found in the peripheral blood; indeed, the former are mainly CD56dull and about a half do not express CD16. Furthermore, these cells are more prompt to be stimulated with IL2 and, unexpectedly, do not lyse autologous hepatocytes, although these cells do not bear HLA class I antigens [reviewed in Ref. (207)]. Autoimmune diseases that hit the liver are mainly represented by AIH, primary biliary cirrhosis (PBC), and primary sclerosing cholangitis (PSC). AIH is characterized by the progressive destruction of the liver parenchyma which eventually leads to cirrhosis and in several instances to hepatic failure and host death. NK cells, together with γδT cells, play a role in the physiopathology of the AIH (208, 209). This is confirmed also in a murine model where administration of poly immune complexes (IC) can induce a strong production of type I IFN and consequent activation of liver NK cells leading to liver destruction with similar histopathologic features found in human AIH (210). In PBC, besides IL17+ cells infiltrating damaged bile ducts, hepatic NK cells active against biliary epithelial cells are found, but it is to be determined whether they are directly involved in the break of immune tolerance characteristic of this disease (207, 211–213). PSC is characterized on one hand by the reduced frequencies of some alleles of inhibiting receptors for HLA-I (214) and on the other by the expression of peculiar alleles of the NKG2D ligand MICA (215); both these molecular events might regulate the NK cell-mediated immune interaction with cholangiocytes.
NK Cells in Lupus Erythematosus Systemicus
Systemic lupus erythematosus is a systemic autoimmune disease characterized by tissue damage mediated mainly through type II and III hypersensitivity. Several autoantibodies are present in SLE patients and it is evident that interaction with cellular antigens can deliver an activating signal to leukocytes bearing Fcγ receptors, as NK cells and monocyte–macrophages, which eventually leads to cell damage and inflammatory cytokine production. In SLE, a reduction of the absolute number of NK cells with an impaired cytolytic activity is reported (20, 216–222) with an imbalance between CD56dull and CD56bright peripheral blood NK cell subsets (223) characterized by an increase of cytokines production (220) and a lower lymphokine activated killer cell activity (219). It is of note that in different systemic autoimmune disorders, as systemic sclerosis (SSc) and anti-neutrophil cytoplasmic antibody-associated vasculitis, the number of CD3−CD56+ NK cells are markedly reduced (224). These findings could be interpreted either as a consequence or as a pathogenic player of the autoimmune disorder. In addition, the NK cell subsets found in the peripheral blood may be considered as the results of the localization of effector cells within target tissues, mainly in the case of systemic autoimmune diseases (24, 76). Recently, it has been reported a prominent reduction of NK cells expressing the DNAM1 activating receptor together with an up-regulation of DNAM1 ligand on plasmocytoid dendritic cells (pDCs) which in turn can mediate NK cell death through type I IFNα (20). Of note, in the MRL-lpr/lpr mice model kidney-infiltrating NK cells express activation antigens and high content of cytotoxic granules, suggesting a possible role in the kidney tissue damage associated with SLE (20). The presence of autoantibodies to inhibitory NK cell receptors and NKG2A (225) can promote excessive NK cell function leading to increased levels of autoantigens and further stimulating autoimmune reactions. Of note, in SLE an increase of CS1/CD319 activating receptor of the SLAM family on NK and pDCs could be detected upon triggering with RNA-IC (225); in addition, expression of CS1/CD319 on B cells of SLE patients increased. Altogether, these findings would suggest a role of CS1/CD319 homophylic interaction among pDC, NK, and B cells in SLE (226, 227). It is still to be determined whether these interactions are involved in the pathogenesis of SLE and whether NK cells may be protective or not in this disease.
Authors’ Viewpoint
It is clear that antigen unspecific autoreactivity can occur, before the onset of an autoimmune disease or in healthy individuals that will not develop any illness: cytotoxic NK (some ILC1 cells), NKT, and γδT cells, are the main active players of this phenomenon while regulatory/tolerant NK cells and ILC2 and ILC3 are mainly involved in maintaining tissue homeostasis. The complex cellular network composed of effector lymphocytes, MSCs, and APCs is the place where the fate of antigen unspecific reactivity determines whether adaptive immune responses will take place or not. One could hypothesize that a strong innate immunity can impede the generation of adaptive immunity as infectious agents are cleared before specific T and B lymphocyte can respond. On the other hand, a low innate response chronically triggers specific T and B cells favoring the establishment of an autoimmune disease due to persistence of the antigen. Finally, an adequate innate response can lead to an optimal B and T cell response that definitively clear the antigen without self-aggression as a consequence (Figure 3). If this idea is true, to cure an autoimmune disease one should trigger innate immunity instead of down-regulate adaptive immunity. However, any therapeutic treatment should take into account that both innate and adaptive immune responses can be regulated through MSCs and EMCs besides lymphocytes and APC.
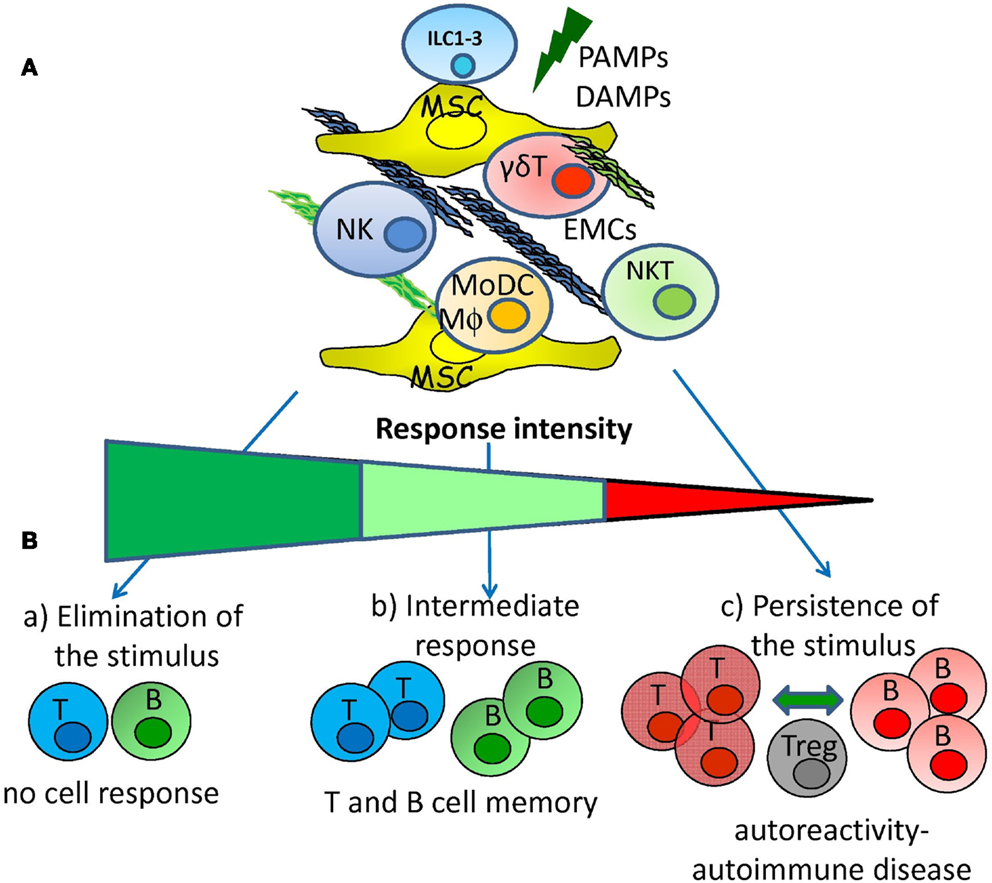
Figure 3. Hypothesis for the generation of adaptive autoreactivity and autoimmunity. (A) Pathogen associated molecular patterns and/or damage associated molecular patterns (PAMPs and DAMPs) can activate innate immunity interacting with receptors expressed on innate lymphoid cells (NK, ILC subsets, NKT, and γδT lymphocytes). The activation of innate immunity can be regulated by reciprocal interactions among mesenchymal stromal cells (MSC), extracellular-matrix components (EMCs), lymphoid cells, monocyte-derived macrophages (MΦ), and dendritic cells (MoDCs). (B) Innate response elicited by NK, ILC subsets, NKT, and γδT lymphocytes interacting with MSC and EMCs can lead to: (a) rapid elimination of the danger signal that avoids the triggering of adaptive immune cell response; (b) intermediate innate response that leads to the triggering of adaptive immunity with the generation of memory T and B cells; (c) low innate response that determines the persistence of the danger signal leading to generation of autoreactive T and B cells. Autoreactive T and B lymphocytes are controlled by regulatory cells (Treg) but chronic stimulation tends to break the tolerance leading to autoimmune disease.
Author Contributions
Both the authors have equally contributed to the preparation and reviewing of this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was partially supported by grants from Associazione Italiana per la Ricerca sul Cancro to Alessandro Poggi (IG 8761) and Maria Raffaella Zocchi (IG 12759) and from Compagnia di San Paolo (2012.0312 ID.ROL 891).
References
1. Trinchieri G. Biology of natural killer cells. Adv Immunol (1989) 47:187–376. doi: 10.1016/S0065-2776(08)60664-1
2. Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol (2004) 5:996–1002. doi:10.1038/ni1114
3. Lanier LL. NK cell recognition. Annu Rev Immunol (2005) 23:225–74. doi:10.1146/annurev.immunol.23.021704.115526
4. Bryceson YT, Long EO. Line of attack: NK cell specifity and integration signal. Curr Opin Immunol (2008) 20:344–52. doi:10.1016/j.coi.2008.03.005
5. Caligiuri MA. Human natural killer cells. Blood (2008) 112:461–9. doi:10.1182/blood-2007-09-077438
6. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Rev Immunol (2008) 9:503–10. doi:10.1038/ni1582
7. Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science (2011) 331:44–9. doi:10.1126/science.1198687
8. Zingoni A, Ardolino M, Santoni A, Cerboni C. NKG2D and DNAM-1 activating receptors and their lignads in NK-T interactions: role in NK cell-mediated negative regulation of T cell responses. Front Immunol (2013) 3:408. doi:10.3389/fimmu.2012.00408
9. Spaggiari GM, Carosio R, Pende D, Marcenaro S, Rivera P, Zocchi MR, et al. NK cell-mediated lysis of autologous antigen-presenting cells is triggered by the engagement of the phosphatidylinositol 3-kinase upon ligation of the natural cytotoxicity receptors NKp30 and NKp46. Eur J Immunol (2001) 31:1656–65. doi:10.1002/1521-4141(200106)31:6<1656::AID-IMMU1656>3.0.CO;2-V
10. Zitvogel L, Terme M, Borg C, Trinchieri G. Dendritic cell-NK cell cross-talk: regulation and physiopathology. Curr Top Microbiol Immunol (2006) 298:157–74.
11. Seaman WE. Natural killer cells and natural killer T cells. Arthritis Rheum (2000) 43:1204–17. doi:10.1002/1529-0131(200006)43:6<1204::AID-ANR3>3.0.CO;2-I
12. Van Kaer L. Natural killer T cells as targets for immunotherapy of autoimmune diseases. Immunol Cell Biol (2004) 82:315–22. doi:10.1111/j.0818-9641.2004.01252.x
13. Bos JD, de Rie MA, Teunissen MB, Piskin G. Psoriasis: dysregulation of innate immunity. Br J Dermatol (2005) 152:1098–107. doi:10.1111/j.1365-2133.2005.06645.x
14. Zhang C, Zhang J, Tian Z. The regulatory effect of natural killer cells: do “NK-reg cells” exist? Cell Mol Immunol (2006) 3:241–54.
15. Lünemann A, Lünemann JD, Münz C. Regulatory NK-cell functions in inflammation and autoimmunity. Mol Med (2009) 15:352–8. doi:10.2119/molmed.2009.00035
16. von Bubnoff D, Andrès E, Hentges F, Bieber T, Michel T, Zimmer J. Natural killer cells in atopic and autoimmune diseases of the skin. J Allergy Clin Immunol (2010) 125:60–8. doi:10.1016/j.jaci.2009.11.020
17. Ahern DJ, Brennan FM. The role of natural killer cells in the pathogenesis of rheumatoid arthritis: major contributors or essential homeostatic modulators? Immunol Lett (2011) 136:115–21. doi:10.1016/j.imlet.2010.11.001
18. Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol (2011) 11:131–42. doi:10.1038/nri2904
19. Conigliaro P, Scrivo R, Valesini G, Perricone R. Emerging role for NK cells in the pathogenesis of inflammatory arthropathies. Autoimmun Rev (2011) 10:577–81. doi:10.1016/j.autrev.2011.04.017
20. Huang Z, Fu B, Zheng SG, Li X, Sun R, Tian Z, et al. Involvement of CD226+ NK cells in immunopathogenesis of systemic lupus erythematosus. J Immunol (2011) 186:3421–31. doi:10.4049/jimmunol.1000569
21. Tian Z, Gershwin ME, Zhang C. Regulatory NK cells in autoimmune disease. J Autoimmun (2012) 39:206–15. doi:10.1016/j.jaut.2012.05.006
22. Zakka LR, Fradkov E, Keskin DB, Tabansky I, Stern JN, Ahmed AR. The role of natural killer cells in autoimmune blistering diseases. Autoimmunity (2012) 45:44–54. doi:10.3109/08916934.2011.606446
23. Chanvillard C, Jacolik RF, Infante-Duarte C, Nayak RC. The role of natural killer cells in multiple sclerosis and their therapeutic implications. Front Immunol (2013) 4:63. doi:10.3389/fimmu.2013.00063
24. Fogel LA, Yokoyama WM, French AR. Natural killer cells in human autoimmune disorders. Arthritis Res Ther (2013) 15:216. doi:10.1186/ar4232
25. Kaur G, Trowsdale J, Fugger L. Natural killer cells and their receptors in multiple sclerosis. Brain (2013) 136:2657–76. doi:10.1093/brain/aws159
26. Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC, et al. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol (1996) 14:619–48. doi:10.1146/annurev.immunol.14.1.619
27. Lanier LL. NK cell receptors. Annu Rev Immunol (1998) 16:359–93. doi:10.1146/annurev.immunol.16.1.359
28. Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol (1999) 17:875–904. doi:10.1146/annurev.immunol.17.1.875
29. Kärre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol (2002) 55:221–8. doi:10.1046/j.1365-3083.2002.01053.x
30. Kärre K. Natural killer cell recognition of missing self. Nat Immunol (2008) 9(5):477–80. doi:10.1038/ni0508-477
31. Moretta A, Poggi A, Pende D, Tripodi G, Orengo AM, Pella N, et al. CD69-mediated pathway of lymphocyte activation: anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic T lymphocytes expressing T cell receptor alpha/beta. J Exp Med (1991) 174:1393–8. doi:10.1084/jem.174.6.1393
32. Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol (2001) 19:197–223. doi:10.1146/annurev.immunol.19.1.197
33. Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev (2010) 235:267–85. doi:10.1111/j.0105-2896.2010.00893.x
34. Meyaard L, Adema GJ, Chang C, Woollatt E, Sutherland GR, Lanier LL, et al. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity (1997) 7:283–90. doi:10.1016/S1074-7613(00)80530-0
35. Waggoner SN, Kumar V. Evolving role of 2B4/CD244 in T and NK cell responses during virus infection. Front Immunol (2012) 3:377. doi:10.3389/fimmu.2012.00377
36. Meinke S, Watzl C. NK cell cytotoxicity mediated by 2B4 and NTB-A is dependent on SAP acting downstream of receptor phosphorylation. Front Immunol (2013) 4:3. doi:10.3389/fimmu.2013.00003
37. Chan CJ, Smyth MJ, Martinet L. Molecular mechanisms of natural killer cell activation to cellular stress. Cell Death Diff (2013) 21(1):5–14. doi:10.1038/cdd.2013.26
38. Flodstrom-Tulberg M, Bryceson YT, Shi F-D, Hoglund P, Ljunggren H-G. Human natural killer cells in human autoimmunity. Curr Opin Immunol (2009) 21:634–40. doi:10.1016/j.coi.2009.09.012
39. Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med (1998) 187:2065–72. doi:10.1084/jem.187.12.2065
40. Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, et al. HIV modulates the expression of lignads important in natural killer cell cytotoxicity on infected primary T cell blasts. Blood (2007) 110:1207–14. doi:10.1182/blood-2006-06-028175
41. Roy S, Barnes PF, Garg A, Wu S, Cosman D, Vankayalapati R. NK cell lyse regulatory T cells that expand in response to an intracellular pathogen. J Immunol (2008) 180:1729–36.
42. Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene (2008) 27:5944–58. doi:10.1038/onc.2008.272
43. Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity (2009) 31:184–96. doi:10.1016/j.immuni.2009.08.006
44. Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells: bridging innate and adaptive immunity. Cell Tissue Res (2011) 343:43–55. doi:10.1007/s00441-010-1023-3
45. Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med (2003) 198:557–67. doi:10.1084/jem.20030788
46. Elishmereni M, Levi-Schaffer F. CD48: a co-stimulatory receptor of immunity. Int J Biochem Cell Biol (2011) 43:25–8. doi:10.1016/j.biocel.2010.09.001
47. Furukawa H, Kitazawa H, Kaneko I, Matsubara M, Nose M, Ono M. Role of 2B4-mediated signals in the pathogenesis of a murine hepatitis model independent of Fas and Valpha14 NKT cells. Immunology (2009) 128:e151–8. doi:10.1111/j.1365-2567.2008.02936.x
48. Loschko J, Krug A. Antigen delivery to plasmacytoid dendritic cells -induction of tolerance and immunity. Crit Rev Immunol (2012) 32:489–501. doi:10.1615/CritRevImmunol.v32.i6.20
49. Münz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med (2005) 202:203–7. doi:10.1084/jem.20050810
50. Guerder S, Joncker N, Mahiddine K, Serre L. Dendritic cells in tolerance and autoimmune diabetes. Curr Opin Immunol (2013) 25(6):670–5. doi:10.1016/j.coi.2013.10.004
51. Galicia G, Gommerman JL. Plasmacytoid dendritic cells and autoimmune inflammation. Biol Chem (2013). doi:10.1515/hsz-2013-0213
52. Trinchieri G. Immunobiology of interleukin-12. Immunol Res (1998) 17:269–78. doi:10.1007/BF02786451
53. Carbone E, Terrazzano G, Ruggiero G, Zanzi D, Ottaiano A, Manzo C, et al. Recognition of autologous dendritic cells by human NK cells. Eur J Immunol (1999) 29:4022–9. doi:10.1002/(SICI)1521-4141(199912)29:12<4022::AID-IMMU4022>3.3.CO;2-F
54. Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Münz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med (2002) 195:343–51. doi:10.1084/jem.20011149
55. Pende D, Castriconi R, Romagnani P, Spaggiari GM, Marcenaro S, Dondero A, et al. Expression of the DNAM-1 ligands nectin-2 (CD112) and poliovirus receptor (CD115), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood (2006) 107:2030–6. doi:10.1182/blood-2005-07-2696
56. Poggi A, Zocchi MR. Antigen presenting cells and stromal cells trigger human natural killer lymphocytes to autoreactivity: evidence for the involvement of natural cytotoxicity receptors (NCR) and NKG2D. Clin Dev Immunol (2006) 13:325–36. doi:10.1080/17402520600578194
57. Poggi A, Prevosto C, Zancolli M, Canevali P, Musso A, Zocchi MR. NKG2D and natural cytotoxicity receptors are involved in natural killer cell interaction with self-antigen presenting cells and stromal cells. Ann N Y Acad Sci (2007) 1109:47–57. doi:10.1196/annals.1398.007
58. Poggi A, Zocchi MR. Human natural killer lymphocytes through the engagement of natural cytotoxicity receptors and NKG2D can trigger self-aggression. Autoimmun Rev (2007) 6:295–9. doi:10.1016/j.autrev.2006.07.003
59. Nedvetzki S, Sowinski S, Eagle RA, Harris J, Vély F, Pende D, et al. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood (2007) 109:3776–85. doi:10.1182/blood-2006-10-052977
60. Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Curr Opin Cell Biol (2012) 24:107–15. doi:10.1016/j.ceb.2011.10.004
61. Dustin ML, Springer TA. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol (1991) 9:27–66. doi:10.1146/annurev.iy.09.040191.000331
62. Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol (2007) 25:619–47. doi:10.1146/annurev.immunol.25.022106.141618
63. Brown AC, Oddos S, Dobbie IM, Alakoskela JM, Parton RM, Eissmann P, et al. Remodelling of cortical actin where lytic granules dock at natural killer cell immune synapses revealed by super-resolution microscopy. PLoS Biol (2011) 9:e1001152. doi:10.1371/journal.pbio.1001152
64. Herter J, Zarbock A. Integrin regulation during leukocyte recruitment. J Immunol (2013) 190:4451–7. doi:10.4049/jimmunol.1203179
65. Liang D, Zuo A, Shao H, Born WK, O’Brien RL, Kaplan HJ, et al. IL-23 receptor expression on γδ T cells correlates with their enhancing or suppressive effects on autoreactive T cells in experimental autoimmune uveitis. J Immunol (2013) 191:1118–25. doi:10.4049/jimmunol.1300626
66. Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev (2014) 13:3–10. doi:10.1016/j.autrev.2013.06.004
67. Ottaviani C, Nasorri F, Bedini C, de Pità O, Girolomoni G, Cavani A. CD56brightCD16(-) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol (2006) 36:118–28. doi:10.1002/eji.200535243
68. Kaufman G, D’Ovidio R, Kaldawy A, Assy B, Ullmann Y, Etzioni A, et al. An unexpected twist in alopecia areata pathogenesis: are NK cells protective and CD49b+ T cells pathogenic? Exp Dermatol (2010) 19:e347–9. doi:10.1111/j.1600-0625.2010.01106.x
69. Ito T, Ito N, Saatoff M, Hashizume H, Fukamizu H, Nickoloff BJ, et al. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermatol (2008) 128:1196–206. doi:10.1038/sj.jid.5701183
70. Batista MD, Ho EL, Kuebler PJ, Milush JM, Lanier LL, Kallas EG, et al. Skewed distribution of natural killer cells in psoriasis skin lesions. Exp Dermatol (2013) 22:64–6. doi:10.1111/exd.12060
71. McElwee KJ, Gilhar A, Tobin DJ, Ramot Y, Sundberg JP, Nakamura M, et al. What causes alopecia areata? Exp Dermatol (2013) 22:609–26. doi:10.1111/exd.12209
72. Kowarik MC, Grummel V, Wemlinger S, Buck D, Weber MS, Berthele A, et al. Immune cell subtyping in the cerebrospinal fluid of patients with neurological diseases. J Neurol (2014) 261(1):130–43. doi:10.1007/s00415-013-7145-2
73. Jiang W, Chai NR, Maric D, Bielekova B. Unexpected role for granzyme K in CD56bright NK cell-mediated immunoregulation of multiple sclerosis. J Immunol (2011) 187:781–90. doi:10.4049/jimmunol.1100789
74. Martínez-Rodríguez JE, López-Botet M, Munteis E, Rio J, Roquer J, Montalban X, et al. Natural killer cell phenotype and clinical response to interferon-beta therapy in multiple sclerosis. Clin Immunol (2011) 141:348–56. doi:10.1016/j.clim.2011.09.006
75. Mayo L, Quintana FJ, Weiner HL. The innate immune system in demyelinating disease. Immunol Rev (2012) 248:170–87. doi:10.1111/j.1600-065X.2012.01135.x
76. Poli A, Kmiecik J, Domingues O, Hentges F, Bléry M, Chekenya M, et al. NK cells in central nervous system disorders. J Immunol (2013) 190:5355–62. doi:10.4049/jimmunol.1203401
77. Hamann I, Dörr J, Glumm R, Chanvillard C, Janssen A, Millward JM, et al. Characterization of natural killer cells in paired CSF and blood samples during neuroinflammation. J Neuroimmunol (2013) 254:165–9. doi:10.1016/j.jneuroim.2012.08.009
78. Sanvito L, Tomita A, Chihara N, Okamoto T, Lin Y, Ogawa M, et al. Increase of Ki-67+ natural killer cells in multiple sclerosis patients treated with interferon-β and interferon-β combined with low-dose oral steroids. J Neuroimmunol (2011) 236:111–7. doi:10.1016/j.jneuroim.2011.05.005
79. Ren J, Feng Z, Lv Z, Chen X, Li J. Natural killer-22 cells in the synovial fluid of patients with rheumatoid arthritis are an innate source of interleukin 22 and tumor necrosis factor-α. J Rheumatol (2011) 38:2112–8. doi:10.3899/jrheum.101377
80. Saito S, Nakashima A, Myojo-Higuma S, Shiozaki A. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J Reprod Immunol (2008) 77:14–22. doi:10.1016/j.jri.2007.04.007
81. Villanueva J, Lee S, Giannini EH, Graham TB, Passo MH, Filipovich A, et al. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res Ther (2005) 7:R30–7. doi:10.1186/ar1551
82. Dalbeth N, Gundle R, Davies RJ, Lee YC, McMichael AJ, Callan MF. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol (2004) 173:6418–26.
83. Saito S, Murakoshi K, Kotake S, Kamatani N, Tomatsu T. Granzyme B induces apoptosis of chondrocytes with natural killer cell-like cytotoxicity in rheumatoid arthritis. J Rheumatol (2008) 35:1932–43.
84. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood (2007) 110:3499–506. doi:10.1182/blood-2007-02-069716
85. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood (2005) 105:1815–22. doi:10.1182/blood-2004-04-1559
86. Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med (2013) 13:856–67. doi:10.2174/1566524011313050016
87. Le Blanc K. Mesenchymal stromal cells: tissue repair and immune modulation. Cytotherapy (2006) 8:559–61. doi:10.1080/14653240601045399
88. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Developmental committee of the european group for blood and marrow transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet (2008) 371:1579–86. doi:10.1016/S0140-6736(08)60690-X
89. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol (2008) 8:726–36. doi:10.1038/nri2395
90. Poggi A, Prevosto C, Massaro AM, Negrini S, Urbani S, Saccardi R, et al. Interaction between human natural killer cells and bone marrow stromal cells induces NK cell triggering. Role of NKp30 and NKG2D receptors. J Immunol (2005) 175:6352–60.
91. Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells (2006) 24:74–85. doi:10.1634/stemcells.2004-0359
92. Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood (2006) 107:1484–90. doi:10.1182/blood-2005-07-2775
93. Spaggiari GM, Moretta L. Cellular and molecular interactions of mesenchymal stem cells in innate immunity. Immunol Cell Biol (2013) 91:27–31. doi:10.1038/icb.2012.62
94. Kim MH, Kitson RP, Albertsson P, Nannmark U, Basse PH, Kuppen PJ, et al. Secreted and membrane-associated matrix metalloproteinases of IL-2-activated NK cells and their inhibitors. J Immunol (2000) 164:5883–9.
95. Albertsson P, Kim MH, Jonges LE, Kitson RP, Kuppen PJ, Johansson BR, et al. Matrix metalloproteinases of human NK cells. In vivo (2000) 14:269–76.
96. Palmieri G, Serra A, De Maria R, Gismondi A, Milella M, Piccoli M, et al. Cross-linking of alpha 4 beta 1 and alpha 5 beta 1 fibronectin receptors enhances natural killer cell cytotoxic activity. J Immunol (1995) 155:5314–22.
97. Gismondi A, Milella M, Palmieri G, Piccoli M, Frati L, Santoni A. Stimulation of protein tyrosine phosphorylation by interaction of NK cells with fibronectin via alpha 4 beta 1 and alpha 5 beta 1. J Immunol (1995) 154:3128–37.
98. Gismondi A, Bisogno L, Mainiero F, Palmieri G, Piccoli M, Frati L, et al. Proline-rich tyrosine kinase-2 activation by beta 1 integrin fibronectin receptor cross-linking and association with paxillin in human natural killer cells. J Immunol (1997) 159:4729–36.
99. Rabinowich H, Lin WC, Amoscato A, Herberman RB, Whiteside TL. Expression of vitronectin receptor on human NK cells and its role in protein phosphorylation, cytokine production, and cell proliferation. J Immunol (1995) 154:1124–35.
100. Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, et al. CD31/PECAM-1 is a ligand for alpha v beta 3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol (1995) 130:451–60. doi:10.1083/jcb.130.2.451
101. Unger ML, Johansson BR, Nannmark U. Migration of IL-2-activated natural killer cells in vitro: influence of extracellular matrix proteins. APMIS (1996) 104:840–7. doi:10.1111/j.1699-0463.1996.tb04950.x
102. Diao H, Iwabuchi K, Li L, Onoe K, Van Kaer L, Kon S, et al. Osteopontin regulates development and function of invariant natural killer T cells. Proc Natl Acad Sci U S A (2008) 105:15884–9. doi:10.1073/pnas.0806089105
103. Chung JW, Kim MS, Piao ZH, Jeong M, Yoon SR, Shin N, et al. Osteopontin promotes the development of natural killer cells from hematopoietic stem cells. Stem Cells (2008) 26:2114–23. doi:10.1634/stemcells.2008-0370
104. Edsparr K, Johansson BR, Goldfarb RH, Basse PH, Nannmark U, Speetjens FM, et al. Human NK cell lines migrate differentially in vitro related to matrix interaction and MMP expression. Immunol Cell Biol (2009) 87:489–95. doi:10.1038/icb.2009.35
105. Zhang T, Liu S, Yang P, Han C, Wang J, Liu J, et al. Fibronectin maintains survival of mouse natural killer (NK) cells via CD11b/Src/beta-catenin pathway. Blood (2009) 114:4081–8. doi:10.1182/blood-2009-05-21988
106. Rabinowich H, Lin WC, Manciule M, Herberman RB, Whiteside TL. Induction of protein tyrosine phosphorylation in human natural killer cells by triggering via alpha 4 beta 1 or alpha 5 beta 1 integrins. Blood (1995) 85:1858–64.
107. Li YQ, Kobayashi M, Kuramitsu Y, Yuan L, Matsushita K, Yagita H, et al. Enhancement of lymphokine-activated killer cell activity by fibronectin. J Immunother (1997) 20:123–30. doi:10.1097/00002371-199703000-00004
108. Albertsson P, Basse PH, Edsparr K, Kim MH, Goldfarb RH, Kitson RP, et al. Differential locomotion of long- and short-term IL-2-activated murine natural killer cells in a model matrix environment. Scand J Immunol (2007) 66:402–9 Erratum in: Scand J Immunol (2007) 66(5):601. Golfarb, RH [corrected to Goldfarb, RH]. doi:10.1111/j.1365-3083.2007.01956.x
109. Xia S, Guo Z, Yao Y, Xu X, Yi H, Xia D, et al. Liver stroma enhances activation of TLR3-triggered NK cells through fibronectin. Mol Immunol (2008) 45:2831–8. doi:10.1016/j.molimm.2008.01.035
110. Meyaard L, Hurenkamp J, Clevers H, Lanier LL, Phillips JH. Leukocyte associated Ig-like receptor-1 functions as an inhibitory receptor on cytotoxic T cells. J Immunol (1999) 162:5800–4.
111. Meyaard L. The inhibitory collagen receptor LAIR-1 (CD305). J Leukoc Biol (2008) 83:799–803. doi:10.1189/jlb.0907609
112. Lebbink RJ, de Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1 J. J Exp Med (2006) 203:1419–25. doi:10.1084/jem.20052554
113. Lebbink RJ, Raynal N, de Ruiter T, Bihan DG, Farndale RW, Meyaard L. Identification of multiple potent binding sites for human leukocyte associated Ig-like receptor LAIR on collagens II and III. Matrix Biol (2009) 28:202–10. doi:10.1016/j.matbio.2009.03.005
114. Jiang L, Barclay AN. New assay to detect low-affinity interactions and characterization of leukocyte receptors for collagen including leukocyte-associated Ig-like receptor-1 (LAIR-1). Eur J Immunol (2009) 39:1167–75. doi:10.1002/eji.200839188
115. Tang X, Narayanan S, Peruzzi G, Apara A, Natarajan K, Margulies DH, et al. A single residue, arginine 65, is critical for the functional interaction of leukocyte-associated inhibitory receptor-1 with collagens. J Immunol (2009) 182:5446–52. doi:10.4049/jimmunol.0804052
116. Poggi A, Pella N, Morelli L, Spada F, Revello V, Sivori S, et al. p40, a novel surface molecule involved in the regulation of the non-major histocompatibility complex-restricted cytolytic activity in humans. Eur J Immunol (1995) 25:369–76. doi:10.1002/eji.1830250210
117. Poggi A, Tomasello E, Revello V, Nanni L, Costa P, Moretta L. p40 molecule regulates NK cell activation mediated by NK receptors for HLA class I antigens and TCR-mediated triggering of T lymphocytes. Int Immunol (1997) 9:1271–9. doi:10.1093/intimm/9.9.1271
118. Leibson PJ. The regulation of lymphocyte activation by inhibitory receptors. Curr Opin Immunol (2004) 16:328–36. doi:10.1016/j.coi.2004.03.006
119. Lebbink RJ, van den Berg MC, de Ruiter T, Raynal N, van Roon JA, Lenting PJ, et al. The soluble leukocyte-associated Ig-like receptor (LAIR)-2 antagonizes the collagen/LAIR-1 inhibitory immune interaction. J Immunol (2008) 180:1662–9.
120. Olde Nordkamp MJ, van Roon JA, Douwes M, de Ruiter T, Urbanus RT, Meyaard L. Enhanced secretion of leukocyte-associated immunoglobulin-like receptor 2 (LAIR-2) and soluble LAIR-1 in rheumatoid arthritis: LAIR-2 is a more efficient antagonist of the LAIR-1-collagen inhibitory interaction than is soluble LAIR-1. Arthritis Rheum (2011) 63:3749–57. doi:10.1002/art.30612
121. Simone R, Pesce G, Antola P, Merlo DF, Bagnasco M, Saverino D. Serum LAIR-2 is increased in autoimmune thyroid diseases. PLoS One (2013) 8(5):e63282. doi:10.1371/journal.pone.0063282
122. Merlo A, Tenca C, Fais F, Battini L, Ciccone E, Grossi CE, et al. Inhibitory receptors CD85j, LAIR-1, and CD152 down-regulate immunoglobulin and cytokine production by human B lymphocytes. Clin Diagn Lab Immunol (2005) 12:705–12.
123. van der Vuurst de Vries AR, Clevers H, Logtenberg T, Meyaard L. Leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) is differentially expressed during human B cell differentiation and inhibits B cell receptor mediated signaling. Eur J Immunol (1999) 29:3160–7. doi:10.1002/(SICI)1521-4141(199910)29:10<3160::AID-IMMU3160>3.0.CO;2-S
124. Jansen CA, Cruijsen CW, de Ruiter T, Nanlohy N, Willems N, Janssen-Korpela PL, et al. Regulated expression of the inhibitory receptor LAIR-1 on human peripheral T cells during T cell activation and differentiation. Eur J Immunol (2007) 37:914–24. doi:10.1002/eji.200636678
125. Poggi A, Tomasello E, Ferrero E, Zocchi MR, Moretta L. p40/LAIR-1 regulates the differentiation of peripheral blood precursors to dendritic cells induced by granulocyte-monocyte colony-stimulating factor. Eur J Immunol (1999) 28:2086–91. doi:10.1002/(SICI)1521-4141(199807)28:07<2086::AID-IMMU2086>3.0.CO;2-T
126. Bonaccorsi I, Cantoni C, Carrega P, Oliveri D, Lui G, Conte R, et al. The immune inhibitory receptor LAIR-1 is highly expressed by plasmacytoid dendritic cells and acts complementary with NKp44 to control IFNα production. PLoS One (2010) 30:e15080. doi:10.1371/journal.pone.0015080
127. Poggi A, Pellegatta F, Leone BE, Moretta L, Zocchi MR. Engagement of the leukocyte associated Ig-like receptor-1 induces programmed cell death and prevents NF-kappaB nuclear translocation in human myeloid leukemias. Eur J Immunol (2000) 30:2751–8. doi:10.1002/1521-4141(200010)30:10<2751::AID-IMMU2751>3.0.CO;2-L
128. Zocchi MR, Pellegatta F, Pierri I, Gobbi M, Poggi A. Leukocyte-associated Ig-like receptor-1 prevents granulocyte-monocyte colony stimulating factor-dependent proliferation and Akt1/PKB alpha activation in primary acute myeloid leukemia cells. Eur J Immunol (2001) 31:3667–75. doi:10.1002/1521-4141(200112)31:12<3667::AID-IMMU3667>3.0.CO;2-G
129. Poggi A, Catellani S, Bruzzone A, Caligaris-Cappio F, Gobbi M, Zocchi MR. Lack of the leukocyte-associated Ig-like receptor-1 expression in high-risk chronic lymphocytic leukaemia results in the absence of a negative signal regulating kinase activation and cell division. Leukemia (2008) 22:980–8. doi:10.1038/leu.2008.21
130. Rygiel TP, Stolte EH, de Ruiter T, van de Weijer ML, Meyaard L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol Immunol (2011) 49:402–6. doi:10.1016/j.molimm.2011.09.006
131. Burns GF, Werkmeister JA, Triglia T. A novel antigenic cell surface protein associated with T200 is involved in the post-activation stage of human NK cell-mediated lysis. J Immunol (1984) 133:1391–6.
132. Colombo BM, Canevali P, Magnani O, Rossi E, Puppo F, Zocchi MR, et al. Defective expression and function of the leukocyte associated Ig-like receptor 1 in B lymphocytes from sistemi lupus erythematosus patients. PLoS One (2012) 7:e31903. doi:10.1371/journal.pone.0031903
133. Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Front Immunol (2013) 4:190. doi:10.3389/fimmu.2013.00190
134. Schmitt EG, Williams CB. Generation and function of induced regulatory T cells. Front Immunol (2013) 4:152. doi:10.3389/fimmu.2013.00152
135. Gianchecchi E, Delfino DV, Fierabracci A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun Rev (2013) 12(11):1091–100. doi:10.1016/j.autrev.2013.05.003
136. Lehtimäki S, Lahesmaa R. Regulatory T cells control immune responses through their non-redundant tissue specific features. Front Immunol (2013) 4:294. doi:10.3389/fimmu.2013.00294
137. Tsai S, Santamaria P. MHC class II polymorphisms, autoreactive T-cells, and autoimmunity. Front Immunol (2013) 4:321. doi:10.3389/fimmu.2013.00321
138. Crome SQ, Lang PA, Lang KS, Ohashi PS. Natural killer cells regulate diverse T cell responses. Trends Immunol (2013) 34:342–9. doi:10.1016/j.it.2013.03.002
139. Sun H, Sun C, Tian Z, Xiao W. NK cells in immunotolerant organs. Cell Mol Immunol (2013) 10:202–12. doi:10.1038/cmi.2013.9
140. Deniz G, Erten G, Kücüksezer UC, Kocacik D, Karagiannidis C, Aktas E, et al. Regulatory NK cells suppress antigen-specific T cell responses. J Immunol (2008) 180:850–7.
141. Ehlers M, Papewalis C, Stenzel W, Jacobs B, Meyer KL, Deenen R, et al. Immunoregulatory natural killer cells suppress autoimmunity by down-regulating antigen-specific CD8+ T cells in mice. Endocrinology (2012) 153:4367–79. doi:10.1210/en.2012-1247
142. Schneidawind D, Pierini A, Negrin RS. Regulatory T cells and natural killer T cells for modulation of GVHD following allogeneic hematopoietic cell transplantation. Blood (2013) 122:3116–21. doi:10.1182/blood-2013-08-453126
143. Wang F, Jiang W, Zhang C, Tian Z, Zhang J. Human natural killer cells exhibit negative regulatory function by ectopic expression of hFoxp3 gene. Transplantation (2013) 95:1324–30. doi:10.1097/TP.0b013e318290b086
144. Fu B, Li X, Sun R, Tong X, Ling B, Tian Z, et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci U S A (2013) 110:E231–40. doi:10.1073/pnas.1206322110
145. Cooper MA, Fehnunger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for CD56bright subset. Blood (2001) 97:3146–51. doi:10.1182/blood.V97.10.3146
146. Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med (2000) 342:1350–8. doi:10.1056/NEJM200005043421807
147. Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity (2001) 14:715–25. doi:10.1016/S1074-7613(01)00147-9
148. Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol (2006) 24:99–146. doi:10.1146/annurev.immunol.24.021605.090737
149. Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD, et al. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc Natl Acad Sci U S A (2007) 104:3378–83. doi:10.1073/pnas.0611098104
150. Spaggiari GM, Contini P, Carosio R, Arvigo M, Ghio M, Oddone D, et al. Soluble HLA class I molecules induce natural killer cell apoptosis through the engagement of CD8: evidence for a negative regulation exerted by members of the inhibitory receptor superfamily. Blood (2002) 99:1706–14. doi:10.1182/blood.V99.5.1706
151. Spaggiari GM, Contini P, Dondero A, Carosio R, Puppo F, Indiveri F, et al. Soluble HLA class I induces NK cell apoptosis upon the engagement of killer-activating HLA class I receptors through FasL-Fas interaction. Blood (2002) 100:4098–107. doi:10.1182/blood-2002-04-1284
152. Ghio M, Contini P, Negrini S, Boero S, Musso A, Poggi A. Soluble HLA-I-mediated secretion of TGF-β1 by human NK cells and consequent down-regulation of anti-tumor cytolytic activity. Eur J Immunol (2009) 39:3459–68. doi:10.1002/eji.200939728
153. Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol (2006) 176:1517–24.
154. Fu B, Wang F, Sun R, Ling B, Tian Z, Wei H. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunology (2011) 133:350–9. doi:10.1111/j.1365-2567.2011.03446.x
155. Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med (2003) 198:1201–12. doi:10.1084/jem.20030305
156. Deniz G, van de Veen W, Akdis M. Natural killer cells in patients with allergic diseases. J Allergy Clin Immunol (2013) 132:527–35. doi:10.1016/j.jaci.2013.07.030
157. Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity (2013) 38:769–81. doi:10.1016/j.immuni.2013.02.010
158. Sciumé G, Hirahara K, Takahashi H, Laurence A, Villarino AV, Singleton KL, et al. Distinct requirements for T-bet in gut innate lymphoid cells. J Exp Med (2012) 209:2331–8. doi:10.1084/jem.20122097
159. Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature (2009) 457:722–5. doi:10.1038/nature07537
160. Fuchs A, Colonna M. Innate lymphoid cells in homeostasis, infection, chronic inflammation and tumors of the gastrointestinal tract. Curr Opin Gastroenterol (2013) 29:581–7. doi:10.1097/MOG.0b013e328365d339
161. Wei H, Zhang J, Xiao W, Feng J, Sun R, Tian Z. Involvement of human natural killer cells in asthma pathogenesis: natural killer 2 cells in type 2 cytokine predominance. J Allergy Clin Immunol (2005) 115:841–7. doi:10.1016/j.jaci.2004.11.026
162. Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med (2006) 203:1851–8. doi:10.1084/jem.20060603
163. Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol (2011) 12:21–7. doi:10.1038/ni.1962
164. Takayama T, Kamada N, Chinen H, Okamoto S, Kitazume MT, Chang J, et al. Imbalance of NKp44(+)NKp46(-) and NKp44(-)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn’s disease. Gastroenterology (2010) 139:.e1–3. doi:10.1053/j.gastro.2010.05.040
165. Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science (2010) 327:1098–102. doi:10.1126/science.1178334
166. Magombedze G, Reddy PB, Eda S, Ganusov VV. Cellular and population plasticity of helper CD4(+) T cell responses. Front Physiol (2013) 4:206. doi:10.3389/fphys.2013.00206
167. Wood KJ, Bushell A, Jones ND. Immunologic unresponsiveness to alloantigen in vivo: a role for regulatory T cells. Immunol Rev (2011) 241:119–32. doi:10.1111/j.1600065X.2011.01013.x
168. Holm SJ, Sakuraba K, Mallbris L, Wolk K, Ståhle M, Sánchez FO. Distinct HLA-C/KIR genotype profile associates with guttate psoriasis. J Invest Dermatol (2005) 125:721–30. doi:10.1111/j.0022-202X.2005.23879.x
169. Williams F, Meenagh A, Sleator C, Cook D, Fernandez-Vina M, Bowcock AM, et al. Activating killer cell immunoglobulin-like receptor gene KIR2DS1 is associated with psoriatic arthritis. Hum Immunol (2005) 66:836–41. doi:10.1016/j.humimm.2005.04.005
170. Płoski R, Luszczek W, Kusnierczyk P, Nockowski P, Cisło M, Krajewski P, et al. A role for KIR gene variants other than KIR2DS1 in conferring susceptibility to psoriasis. Hum Immunol (2006) 67:521–6. doi:10.1016/j.humimm.2006.04.001
171. Yen JH, Lin CH, Tsai WC, Wu CC, Ou TT, Hu CJ, et al. Killer cell immunoglobulin-like receptor gene’s repertoire in rheumatoid arthritis. Scand J Rheumatol (2006) 35:124–7. doi:10.1080/03009740500381252
172. Hou YF, Zhang YC, Jiao YL, Wang LC, Li JF, Pan ZL, et al. Disparate distribution of activating and inhibitory killer cell immunoglobulin-like receptor genes in patients with systemic lupus erythematosus. Lupus (2010) 19:20–6. doi:10.1177/0961203309345779
173. Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature (2010) 466:113–7. doi:10.1038/nature09114
174. Jelcic I, Hsu KC, Kakalacheva K, Breiden P, Dupont B, Uhrberg M, et al. Killer immunoglobulin-like receptor locus polymorphisms in multiple sclerosis. Mult Scler (2012) 18:951–8. doi:10.1177/1352458511431726
175. Ramírez-De los Santos S, Sánchez-Hernández PE, Muñoz-Valle JF, Palafox-Sánchez CA, Rosales-Rivera LY, García-Iglesias T, et al. Associations of killer cell immunoglobulin-like receptor genes with rheumatoid arthritis. Dis Markers (2012) 33:201–6. doi:10.3233/DMA-2012-0927
176. Kusnierczyk P. Killer cell immunoglobulin-like receptor gene associations with autoimmune and allergic diseases, recurrent spontaneous abortion, and neoplasms. Front Immunol (2013) 4:8. doi:10.3389/fimmu.2013.00008
177. Park KS, Park JH, Song YW. Inhibitory NKG2A and activating NKG2D and NKG2C natural killer cell receptor genes: susceptibility for rheumatoid arthritis. Tissue Antigens (2008) 72:342–6. doi:10.1111/j.1399-0039.2008.01110.x
178. Chandran V, Bull SB, Pellett FJ, Ayearst R, Pollock RA, Gladman DD. Killer-cell immunoglobulin-like receptor gene polymorphisms and susceptibility to psoriatic arthritis. Rheumatology (Oxford) (2014) 53(2):233–9. doi:10.1093/rheumatology/ket296
179. Chewning JH, Gudme CN, Hsu KC, Selvakumar A, Dupont B. KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. J Immunol (2007) 179:854–68.
180. Foley B, De Santis D, Lathbury L, Christiansen F, Witt C. KIR2DS1-mediated activation overrides NKG2A-mediated inhibition in HLA-C C2-negative individuals. Int Immunol (2008) 20:555–63. doi:10.1093/intimm/dxn013
181. Sivori S, Carlomagno S, Falco M, Romeo E, Moretta L, Moretta A. Natural killer cells expressing the KIR2DS1-activating receptor efficiently kill T-cell blasts and dendritic cells: implications in haploidentical HSCT. Blood (2011) 117:4284–92. doi:10.1182/blood-2010-10-316125
182. Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med (2012) 367:805–16. doi:10.1056/NEJMoa1200503
183. Ouyang W. Distinct roles of IL-22 in human psoriasis and inflammatory bowel disease. Cytokine Growth Factor Rev (2010) 21:435–41. doi:10.1016/j.cytogfr.2010.10.007
184. Dunphy S, Gardiner CM. NK cells and psoriasis. J Biomed Biotechnol (2011) 2011:248317. doi:10.1155/2011/248317
185. McAleer JP, Kolls JK. Mechanisms controlling Th17 cytokine expression and host defense. J Leukoc Biol (2011) 90:263–70. doi:10.1189/jlb.0211099
186. Wolk K, Witte E, Warszawska K, Schulze-Tanzil G, Witte K, Philipp S, et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis. Eur J Immunol (2009) 39:3570–81. doi:10.1002/eji.200939687
187. Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol (2008) 159:1092–102. doi:10.1111/j.1365-2133.2008.08769.x
188. Patel F, Marusina AI, Duong C, Adamopoulos IE, Maverakis E. NKG2C, HLA-E and their association with psoriasis. Exp Dermatol (2013) 22:797–9. doi:10.1111/exd.12280
189. Zeng X, Chen H, Gupta R, Paz-Altschul O, Bowcock AM, Liao W. Deletion of the activating NKG2C receptor and a functional polymorphism in its ligand HLA-E in psoriasis susceptibility. Exp Dermatol (2013) 22:679–81. doi:10.1111/exd.12233
190. Takahashi H, Amagai M, Tanikawa A, Suzuki S, Ikeda Y, Nishikawa T, et al. T helper type 2-biased natural killer cell phenotype in patients with pemphigus vulgaris. J Invest Dermatol (2007) 127:324–30. doi:10.1038/sj.jid.5700527
191. Stern JN, Keskin DB, Barteneva N, Zuniga J, Yunis EJ, Ahmed AR. Possible role of natural killer cells in pemphigus vulgaris – preliminary observations. Clin Exp Immunol (2008) 152:472–81. doi:10.1111/j.1365-2249.2008.03638.x
192. Benczur M, Petrányl GG, Pálffy G, Varga M, Tálas M, Kotsy B, et al. Dysfunction of natural killer cells in multiple sclerosis: a possible pathogenetic factor. Clin Exp Immunol (1980) 39:657–62.
193. Kastrukoff LF, Lau A, Wee R, Zecchini D, Whit R, Paty DW. Clinical relapses of multiple sclerosis are associated with ‘novel’ valleys in natural killer cell functional activity. J Neuroimmunol (2003) 145:103–14. doi:10.1016/j.jneuroim.2003.10.001
194. Saraste M, Irjala H, Airas L. Expansion of CD56Bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon-beta. Neurol Sci (2007) 28:121–6. doi:10.1007/s10072-007-0803-3
195. Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A (2006) 103:5941–6. doi:10.1073/pnas.0601335103
196. Bielekova B, Howard T, Packer AN, Richert N, Blevins G, Ohayon J, et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch Neurol (2009) 66:483–9. doi:10.1001/archneurol.2009.50
197. Takahashi K, Miyake S, Kondo T, Terao K, Hatakenaka M, Hashimoto S, et al. Natural killer type 2 bias in remission of multiple sclerosis. J Clin Invest (2001) 107:R23–9. doi:10.1172/JCI11819
198. Takahashi K, Aranami T, Endoh M, Miyake S, Yamamura T. The regulatory role of natural killer cells in multiple sclerosis. Brain (2004) 127:1917–27. doi:10.1093/brain/awh219
199. Rodacki M, Svoren B, Butty V, Besse W, Laffel L, Benoist C, et al. Altered natural killer cells in type 1 diabetic patients. Diabetes (2007) 56:177–85. doi:10.2337/db06-0493
200. Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A (2007) 104:5115–20. doi:10.1073/pnas.0700442104
201. Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol (2009) 155:173–81. doi:10.1111/j.1365-2249.2008.03860.x
202. Zhou M, McPherson L, Feng D, Song A, Dong C, Lyu SC, et al. Kruppel-like transcription factor 13 regulates T lymphocyte survival in vivo. J Immunol (2007) 178:5496–504.
203. Chen Y, Hu Y, Zhou T, Zhou KK, Mott R, Wu M, et al. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am J Pathol (2009) 175:2676–85. doi:10.2353/ajpath.2009.080945
204. Perricone R, Perricone C, De Carolis C, Shoenfeld Y. NK cells in autoimmunity: a two-edg’d weapon of the immune system. Autoimmun Rev (2008) 7:384–90. doi:10.1016/j.autrev.2008.03.002
205. Kanai T, Mikami Y, Sujino T, Hisamatsu T, Hibi T. RORγt-dependent IL-17A-producing cells in the pathogenesis of intestinal inflammation. Mucosal Immunol (2012) 5:240–7. doi:10.1038/mi.2012.6
206. Geremia A, Arancibia-Cárcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med (2011) 208(6):1127–33. doi:10.1084/jem.20101712
207. Hudspeth K, Pontarini E, Tentorio P, Cimino M, Donadon M, Torzilli G, et al. The role of natural killer cells in autoimmune liver disease: a comprehensive review. J Autoimmun (2013) 46:55–65. doi:10.1016/j.jaut.2013.07.003
208. Strassburg CP, Obermayer-Straub P, Manns MP. Autoimmunity in liver diseases. Clin Rev Allergy Immunol (2000) 18:127–39. doi:10.1385/CRIAI:18:2:127
209. Oo YH, Hubscher SG, Adams DH. Autoimmune hepatitis: new paradigms in the pathogenesis, diagnosis, and management. Hepatol Int (2010) 4:475–93. doi:10.1007/s12072-010-9183-5
210. Chen Y, Wei H, Gao B, Hu Z, Zheng S, Tian Z. Activation and function of hepatic NK cells in hepatitis B infection: an underinvestigated innate immune response. J Viral Hepat (2005) 12:38–45. doi:10.1111/j.1365-2893.2005.00543.x
211. Harada K, Shimoda S, Sato Y, Isse K, Ikeda H, Nakanuma Y. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol (2009) 157:261–70. doi:10.1111/j.1365-2249.2009.03947.x
212. Shimoda S, Harada K, Niiro H, Shirabe K, Taketomi A, Maehara Y, et al. Interaction between Toll-like receptors and natural killer cells in the destruction of bile ducts in primary biliary cirrhosis. Hepatology (2011) 53(4):1270–81. doi:10.1002/hep.24194
213. Shimoda S, Tsuneyama K, Kikuchi K, Harada K, Nakanuma Y, Nakamura M, et al. The role of natural killer (NK) and NK T cells in the loss of tolerance in murine primary biliary cirrhosis. Clin Exp Immunol (2012) 168:279–84. doi:10.1111/j.1365-2249.2012.04581.x
214. Karlsen TH, Boberg KM, Olsson M, Sun JY, Senitzer D, Bergquist A, et al. Particular genetic variants of ligands for natural killer cell receptors may contribute to the HLA associated risk of primary sclerosing cholangitis. J Hepatol (2007) 46:899–906. doi:10.1016/j.jhep.2007.01.032
215. Van Steenbergen W, De Goede E, Emonds MP, Reinders J, Tilanus M, Fevery J. Primary sclerosing cholangitis in two brothers: report of a family with special emphasis on molecular HLA and MICA genotyping. Eur J Gastroenterol Hepatol (2005) 17:767–71. doi:10.1097/00042737-200507000-00013
216. Riccieri V, Spadaro A, Parisi G, Taccari E, Moretti T, Bernardini G, et al. Down-regulation of natural killer cells and of gamma/delta T cells in systemic lupus erythematosus. Does it correlate to autoimmunity and to laboratory indices of disease activity? Lupus (2000) 9:333–7. doi:10.1191/096120300678828460
217. Toyabe S, Kaneko U, Uchiyama M. Decreased DAP12 expression in natural killer lymphocytes from patients with systemic lupus erythematosus is associated with increased transcript mutations. J Autoimmun (2004) 23:371–8. doi:10.1016/j.jaut.2004.09.003
218. Green MR, Kennell AS, Larche MJ, Seifert MH, Isenberg DA, Salaman MR. Natural killer cell activity in families of patients with systemic lupus erythematosus: demonstration of a killing defect in patients. Clin Exp Immunol (2005) 14:165–73. doi:10.1111/j.1365-2249.2009.03938.x
219. Park YW, Kee SJ, Cho YN, Lee EH, Lee HY, Kim EM, et al. Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum (2009) 60:1753–63. doi:10.1002/art.24556
220. Hervier B, Beziat V, Haroche J, Mathian A, Lebon P, Ghillani-Dalbin P, et al. Phenotype and function of natural killer cells in systemic lupus erythematosus: excess interferon-γ production in patients with active disease. Arthritis Rheum (2011) 63:1698–706. doi:10.1002/art.30313
221. Abud-Mendoza C, Cuevas-Orta E, Santillán-Guerrero EN, Martínez-Martínez MU, Hernández-Castro B, Estrada-Capetillo L, et al. Decreased blood levels of B lymphocytes and NK cells in patients with systemic lupus erythematosus (SLE) infected with papillomavirus (HPV). Arch Dermatol Res (2013) 305:117–23. doi:10.1007/s00403-012-1258-9
222. Henriques A, Teixeira L, Inês L, Carvalheiro T, Gonçalves A, Martinho A, et al. NK cells dysfunction in systemic lupus erythematosus: relation to disease activity. Clin Rheumatol (2013) 32:805–13. doi:10.1007/s10067-013-2176-8
223. Schepis D, Gunnarsson I, Eloranta ML, Lampa J, Jacobson SH, Kärre K, et al. Increased proportion of CD56bright natural killer cells in active and inactive systemic lupus erythematosus. Immunology (2009) 126:140–6. doi:10.1111/j.1365-2567.2008.02887.x
224. Puxeddu I, Bongiorni F, Chimenti D, Bombardieri S, Moretta A, Bottino C, et al. Cell surface expression of activating receptors and co-receptors on peripheral blood NK cells in systemic autoimmune diseases. Scand J Rheumatol (2012) 41:298–304. doi:10.3109/03009742.2011.648657
225. Hagberg N, Theorell J, Eloranta ML, Pascal V, Bryceson YT, Rönnblom L. Anti-NKG2A autoantibodies in a patient with systemic lupus erythematosus. Rheumatology (Oxford) (2013) 52:1818–23. doi:10.1093/rheumatology/ket220
226. Hagberg N, Theorell J, Schlums H, Eloranta ML, Bryceson YT, Rönnblom L. Systemic lupus erythematosus immune complexes increase the expression of SLAM family members CD319 (CRACC) and CD229 (LY-9) on plasmacytoid dendritic cells and CD319 on CD56(dim) NK cells. J Immunol (2013) 191:2989–98. doi:10.4049/jimmunol.1301022
Keywords: NK cells, autoreactivity, autoimmunity, NKG2D, DNAM1, regulatory NK cells, mesenchymal stromal cells, LAIR1
Citation: Poggi A and Zocchi MR (2014) NK cell autoreactivity and autoimmune diseases. Front. Immunol. 5:27. doi: 10.3389/fimmu.2014.00027
Received: 13 November 2013; Accepted: 17 January 2014;
Published online: 04 February 2014.
Edited by:
Massimo Vitale, IRCCS AOU San Martino-IST, ItalyReviewed by:
Kasper Hoebe, Cincinnati Children’s Hospital Medical Center, USAAntonio Uccelli, University of Genoa, Italy
Copyright: © 2014 Poggi and Zocchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Poggi, Molecular Oncology and Angiogenesis Unit, IRCCS AOU San Martino-IST, Largo R. Benzi 10, Genoa 16132, Italy e-mail: alessandro.poggi@hsanmartino.it; alessandropoggi1959@gmail.com
 Alessandro Poggi
Alessandro Poggi Maria Raffaella Zocchi
Maria Raffaella Zocchi