- 1Hangzhou Institute of Cardiovascular Diseases, Affiliated Hospital of Hangzhou Normal University, Hangzhou Normal University, Hangzhou, China
- 2Department of Liver Diseases, Ma’anshan Fourth People’s Hospital, Ma’anshan, China
- 3Department of Molecular and Cellular Physiology, Shinshu University School of Medicine, Nagano, Japan
- 4School of Public Health, Hangzhou Medical College, Hangzhou, China
- 5Department of Neurology, Affiliated Hospital of Guizhou Medical University, Guiyang, China
Background: Metabolic dysfunction-associated fatty liver disease [MAFLD, formerly known as nonalcoholic fatty liver disease (NAFLD)] is one of the most important causes of liver disease worldwide, while cardiovascular disease (CVD) is still one of the main causes of morbidity and mortality worldwide, and the two are closely related. This study aimed to investigate the risk of CVD incidence or CVD-related mortality (CVD mortality) in patients diagnosed with MAFLD under new concepts and new diagnostic criteria.
Methods: We searched English databases PubMed, Web of Science, Embase, and Cochrane Library for relevant literature. The language was restricted to English.
Results: By 22 January 2022, 556 published studies were obtained through preliminary retrieval, and 10 cohort studies were included in this study. All statistical analyses were performed using Review Manager 5.2 software. Compared with the control group, patients in the MAFLD group had a significantly higher relative risk of CVD incidence or CVD mortality during the follow-up, with an RR rate of 1.95 (95% CI 1.76–2.17, p < 0.01). The incidence of CVD in the MAFLD group was more than twice that in the control group (RR 2.26, 95% CI 2.00–2.54, p < 0.01). The mortality rate of CVD was 1.57 times higher than that in the control group (RR 1.57, 95% CI 1.42–1.72, p < 0.01).
Conclusions: Patients diagnosed with MAFLD alone had higher cardiovascular mortality than those diagnosed with NAFLD alone based on the available data.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the most important causes of liver disease worldwide, affecting many children and adults worldwide. Existing data indicated that the prevalence of NAFLD in adults worldwide was 25% [95% confidence interval (CI) 22.10–28.65], with the highest reported prevalence in the Middle East (32%) and South America (31%), followed by Asia (27%), the United States (24%), and Europe (23%), and the lowest in Africa (14%) (1, 2). NAFLD is a multi-system disease that not only affects the structure and function of the liver but also increases the incidence of type 2 diabetes, cardiovascular disease (CVD), cerebrovascular disease, and chronic kidney disease (3–5). In addition, NAFLD is believed to be closely associated with an increase in CVD-related mortality (6–8). However, NAFLD is an “exclusive” diagnosis that exists only in the absence of viral hepatitis, autoimmune diseases, or alcohol consumption (9). In 2020, experts reached a consensus to recommend a more appropriate term to more accurately and positively define fatty liver disease associated with metabolic disorders, namely, metabolic dysfunction-associated fatty liver disease (MAFLD) (10, 11). What used to be known as NAFLD has now been renamed MAFLD, a new definition that clearly identifies the disease as a metabolic disorder. In order to meet the diagnosis of MAFLD, patients should have hepatic steatosis (histological, radiological, blood marker, or evidential fraction of fat accumulation) with one of the three characteristics: overweight or obese (cutoff based on race), type 2 diabetes mellitus (T2D), or signs of metabolic disorders (9). Once these criteria are met, a diagnosis of MAFLD is no longer required to exclude any other cause of liver disease.
MAFLD resulted from increased liver fat deposition due to metabolic disorders and was one of many disease entities associated with metabolic disorders (12). People with fatty liver were at increased risk of extrahepatic complications, including CVD and cancer, due to the close association of MAFLD with metabolic disorders (3, 13). A study by Niriella et al. showed that patients in the MAFLD group had a significantly higher risk of cardiovascular events compared with the control group, with 43 of 692 patients diagnosed with MAFLD developing cardiovascular events during the follow-up (14). Another national cohort study indicated a multivariate-adjusted hazard ratio (95% CI) of 1.43 (1.41–1.45) for CVD events in the MAFLD-only group. Lee et al. noted that a change in criteria from NAFLD to acute fatty liver disease might identify more individuals with metabolically complex fatty liver disease and increased risk of CVD (15).
CVD was still one of the leading causes of morbidity and mortality worldwide, accounting for approximately 31.5% of deaths worldwide and 45% of deaths from noncommunicable diseases (16–18). CVD shared many risk factors with MAFLD, including diabetes, abnormal lipid metabolism, hypertension, insulin resistance, and inflammation (9, 19). The possible association between MAFLD and CVD was unclear. The meta-analysis of the association between MAFLD and CVD under the new diagnostic criteria was lacking. Therefore, we conducted a meta-analysis to investigate the risk of CVD incidence or CVD mortality in patients diagnosed with MAFLD.
Materials and methods
Search strategy
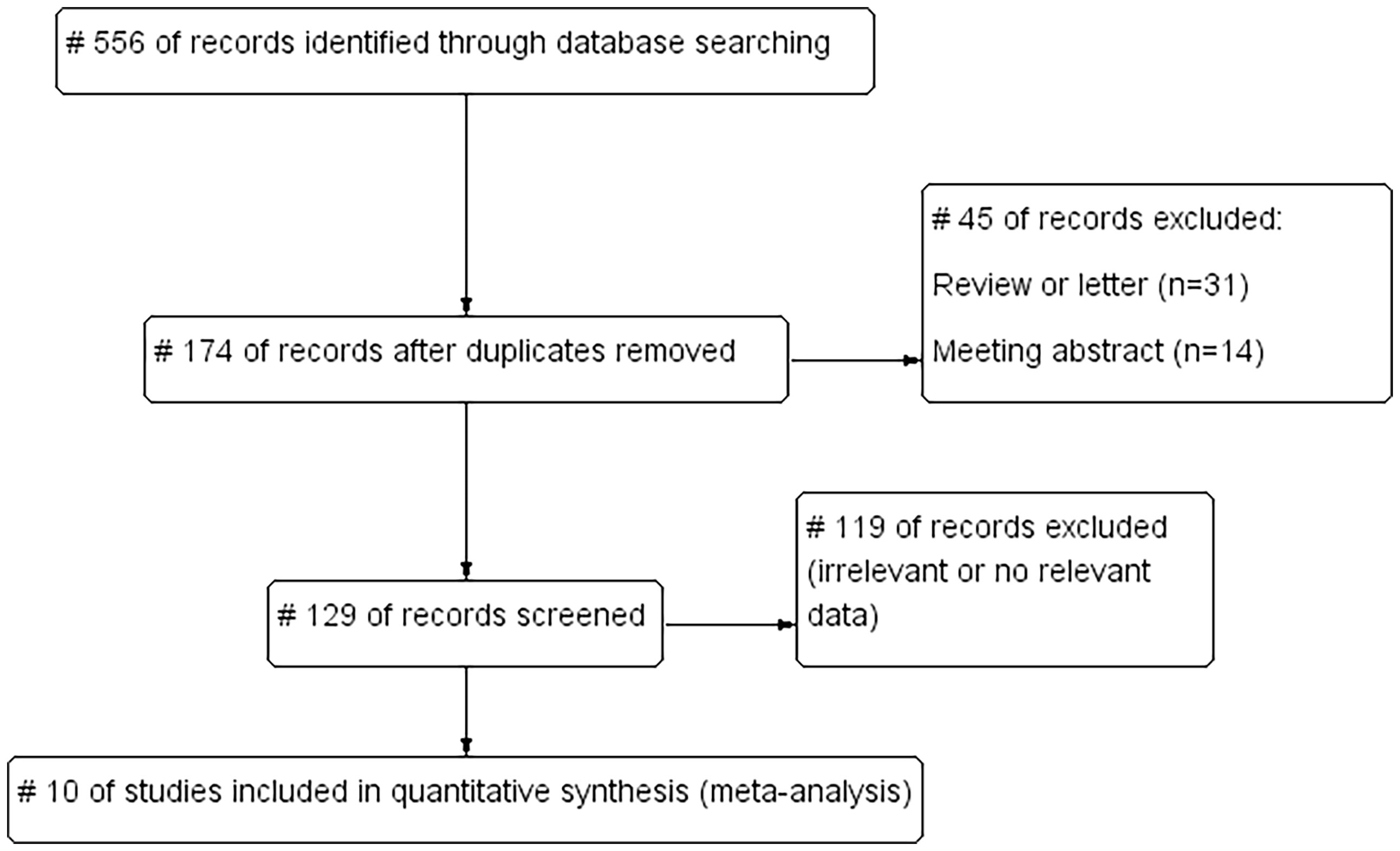
We searched English databases PubMed, Web of Science, Embase, and Cochrane Library for relevant literature. Meanwhile, we screened the references of the retrieved published studies and restricted the language to English. As of 22 January 2022, 556 studies were obtained. After preliminary screening, the literature included were further screened by reading the full text.
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) studies reporting the number or percentage of patients diagnosed with MAFLD who developed CVD or died of CVD during the follow-up in the control population; (2) English studies; and (3) cohort studies.
Exclusion criteria were as follows: (1) summary, conference abstract, or letter; (2) studies with no relevant data; and (3) repeated studies.
Data extraction
A total of 10 studies were included in this analysis, and the outcome indicators observed were the incidence of CVD or CVD mortality during the follow-up period. The main CVDs included coronary heart disease (CHD), myocardial infarction (MI), heart failure (HF), cerebrovascular disease, stroke, and transient ischemic attack (TIA) (20). Data were collected, including the name of the first author, the year of publication, and the number of patients who developed CVD or died of CVD during the follow-up in the MAFLD and control groups. The experimental group (MAFLD group) in this study included patients diagnosed with both MAFLD and NAFLD and patients diagnosed with MAFLD only. The control group (non-MAFLD group) consisted of patients who were not diagnosed with both MAFLD and NAFLD and patients who were not diagnosed with MAFLD but were diagnosed with NAFLD. In addition, we collected data on patients diagnosed only with MAFLD or only with NAFLD.
Statistical analysis
All statistical analyses were performed using Review Manager 5.2 software. A binary controlled study was used to calculate the relative risk of CVD or death from CVD during follow-up compared with the control group. Furthermore, 95% CIs and risk ratios (RRs) were used to measure effect. The results of all studies were aggregated using a random-effects model. Funnel plots were used to assess publication bias.
Results
Outcomes of the electronic search
We searched the English databases PubMed, Web of Science, Embase, and Cochrane Library, and screened the references of the retrieved published studies. The retrieval deadline was 22 January 2022. The search words included “Metabolic Dysfunction–Associated Fatty Liver Disease (MAFLD),” “cardiovascular disease (CVD),” “coronary heart disease (CHD),” “myocardial infarction (MI),” “heart failure (HF),” “cerebrovascular disease,” “stroke,” and “transient ischemic attack (TIA),” and limited the retrieval language to English. A total of 556 studies were obtained in the preliminary retrieval. Also, 382 duplicated studies, 31 reviews or letters, 14 conference abstracts, and 119 studies with irrelevant or no relevant data were excluded, and 10 studies were included (Figure 1).
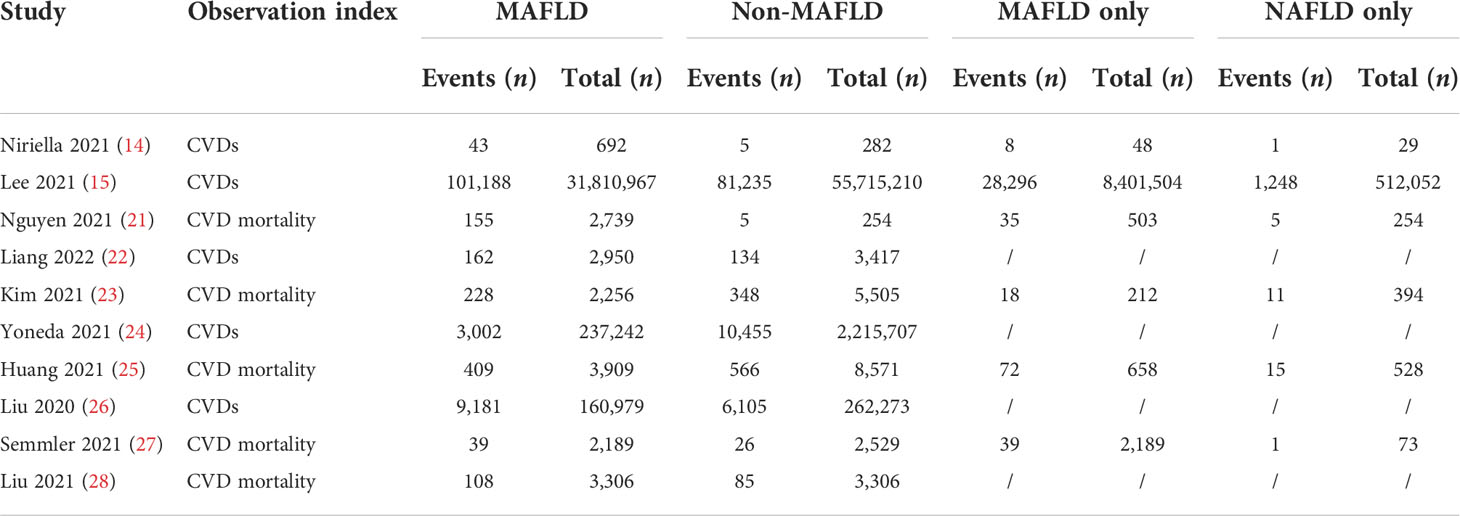
Characteristics of the included studies
The data included in this study were all from cohort studies. The characteristics of all published studies are shown in Table 1. Five of the included articles described the specific number of CVD cases in patients with MAFLD and non-MAFLD during the follow-up, and the other five articles described the specific number of CVD mortality in the two groups during the follow-up. An additional 32,227,229 patients were included in the MAFLD group, of whom 113,576 developed CVD during the follow-up and 939 died of CVD. The control group included 58,217,054 patients, of whom 97,934 developed CVD and 1,030 died of CVD. In addition, 6 of the 10 studies specifically analyzed the follow-up in patients diagnosed only with MAFLD or NAFLD. Of the 8,405 people, 114 were diagnosed with MAFLD alone; 28,304 had CVD, and 164 died of CVD. Among 513,330 patients diagnosed with NAFLD alone, 1,249 developed CVD and 32 died of CVD.
Meta-analysis
This study found that patients in the MAFLD group had a significantly increased relative risk of CVD or death from CVD during the follow-up compared with the control group, with an RR of 1.95 (95% CI 1.76–2.17; I2 = 96%, p < 0.01) (Figure 2), indicating that the incidence of CVD or CVD mortality was 1.95 times higher in the MAFLD group than in the control group. We performed a subgroup analysis and found that the incidence of CVD in the MAFLD group was more than twice that in the control group, with an RR of 2.26 (95% CI 2.00–2.54; I2 = 97%, p < 0.01); CVD mortality was 1.57 times higher than that in the control group (RR 1.57, 95% CI 1.42–1.72; I2 = 5%, p < 0.01) (Figure 3).
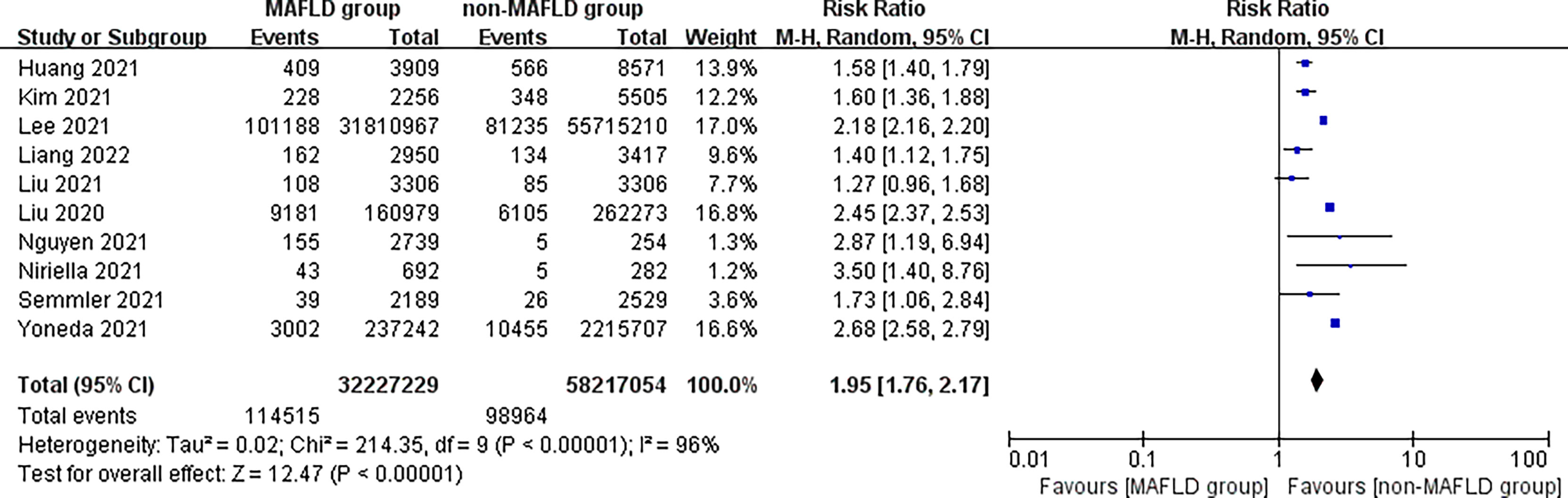
Figure 2 Overall relative risk of CVD incidence or CVD mortality during follow-up between the MAFLD group and the control group.
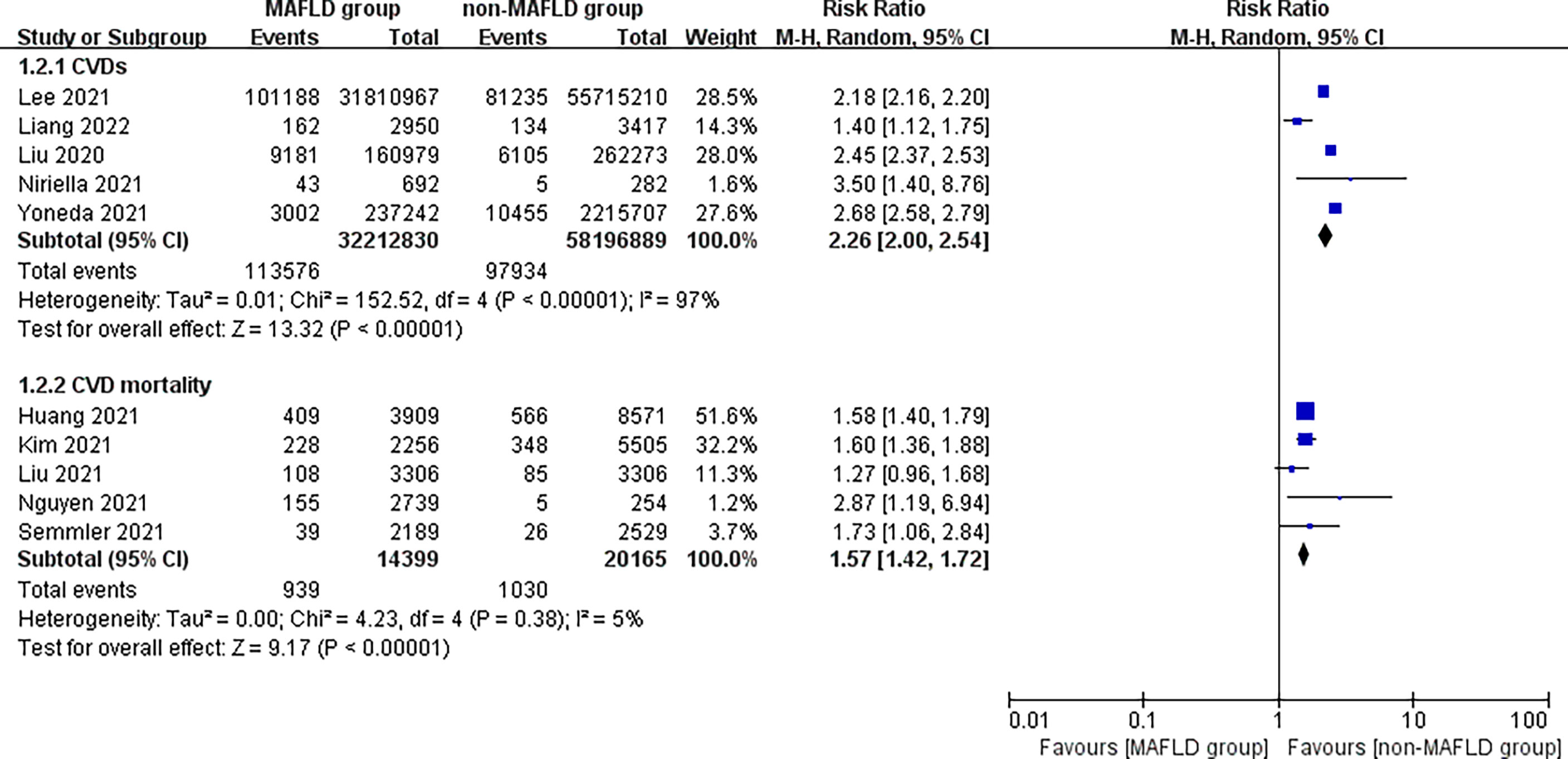
Figure 3 Subgroup analysis of CVD incidence and CVD mortality during follow-up between the MAFLD group and the control group.
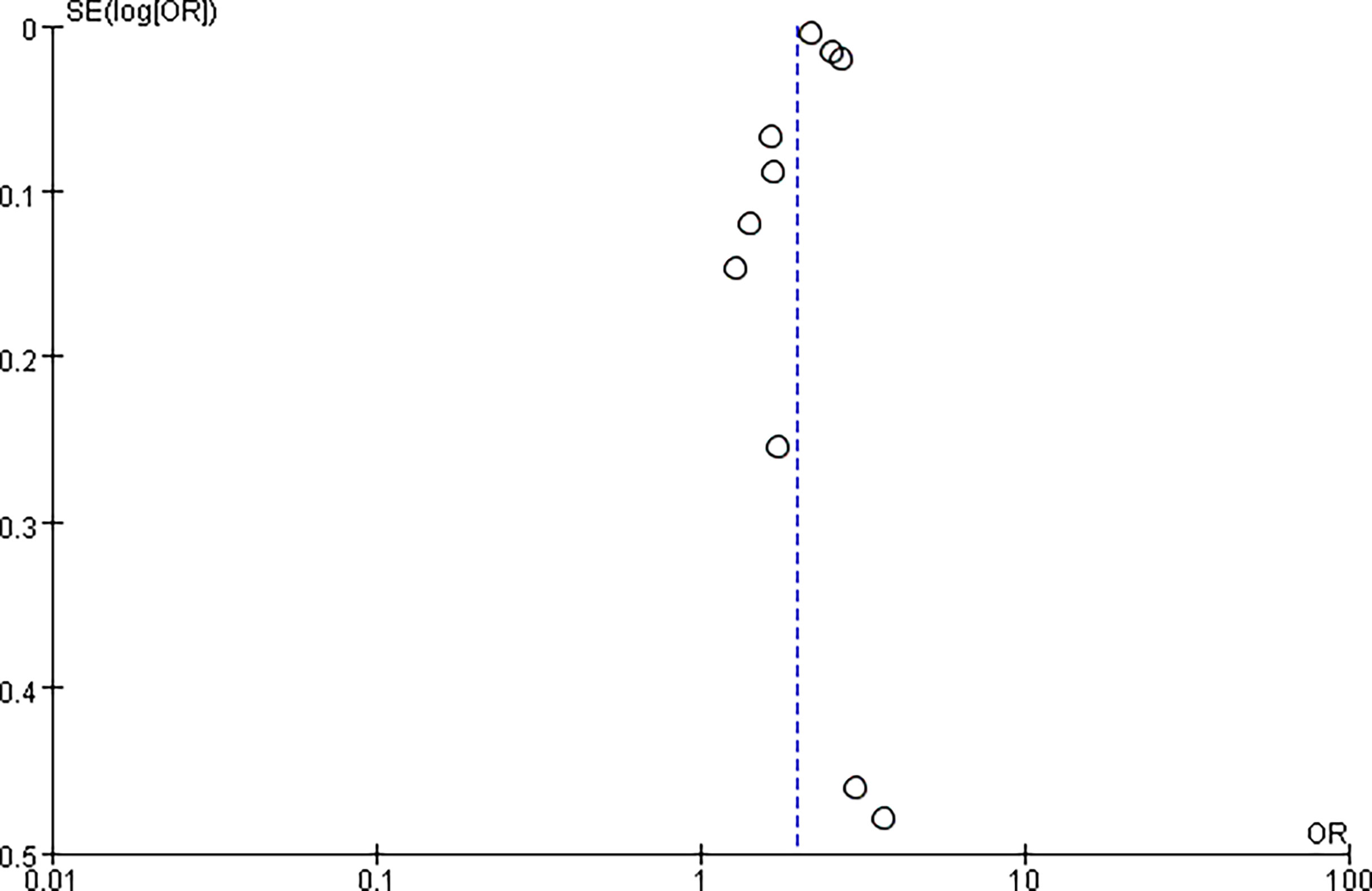
In addition, we separately analyzed the follow-up of patients diagnosed only with MAFLD and those diagnosed only with NAFLD. The risk of CVD or death from CVD was significantly higher in the MAFLD-only group than in the NAFLD-only group, with an RR of 2.57 (95% CI 1.41–4.71; I2 = 78%, P = 0.002) (Figure 4). Our study found no statistical difference in the incidence of CVD between the two groups, with an RR of 1.69 (95% CI 0.69–4.14; I2 = 32%, P = 0.25). However, the cardiovascular mortality was 3.41 times higher in the MAFLD-only group than in the NAFLD-only group (RR 3.41, 95% CI 2.31–5.02; I2 = 0%, p < 0.01) (Figure 5). Funnel diagram shows that there is no significant publication bias in the included articles (Figure 6).
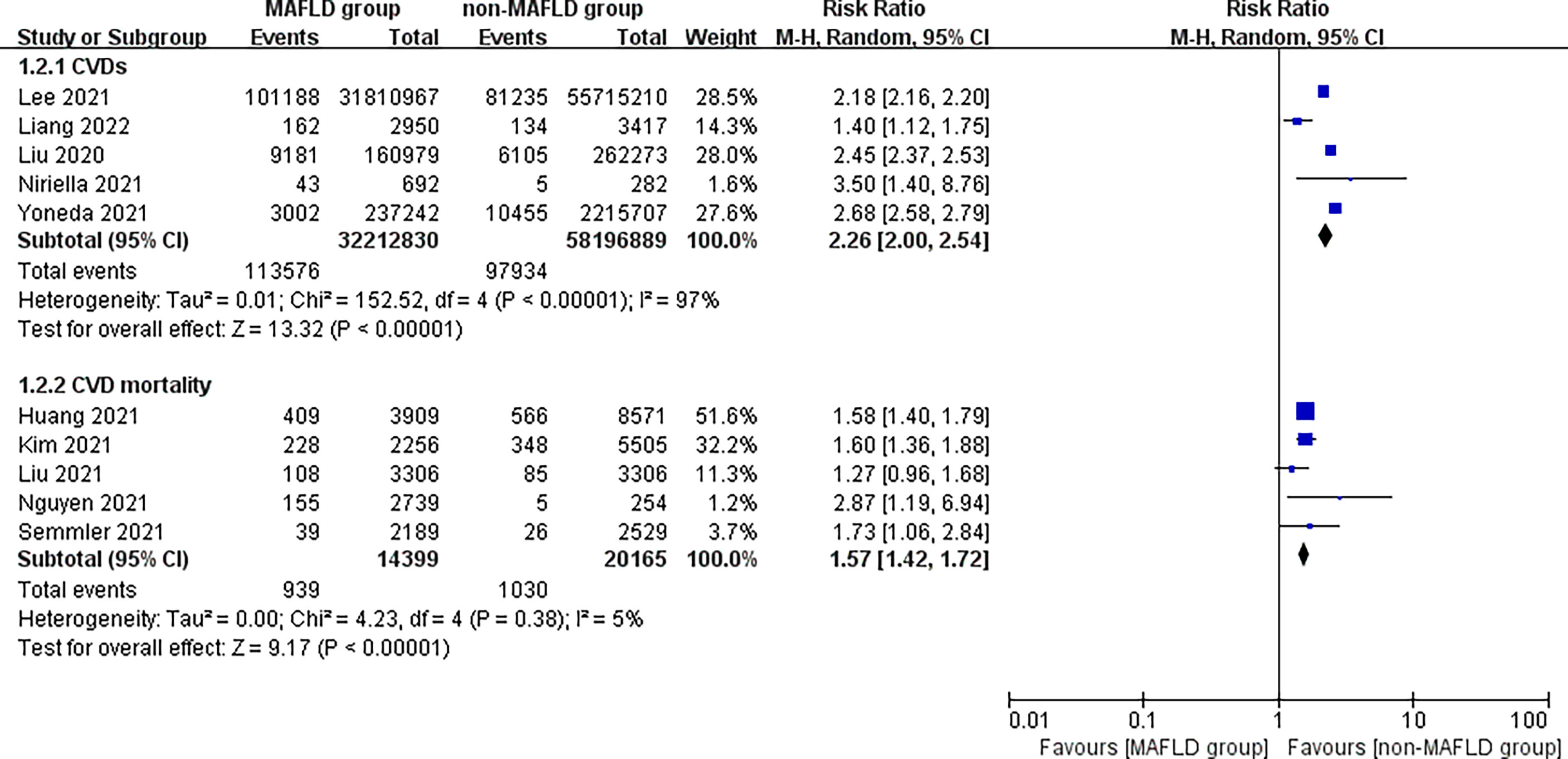
Figure 4 Overall relative risk of CVD incidence and CVD mortality during follow-up between the MAFLD-only and NAFLD-only groups.
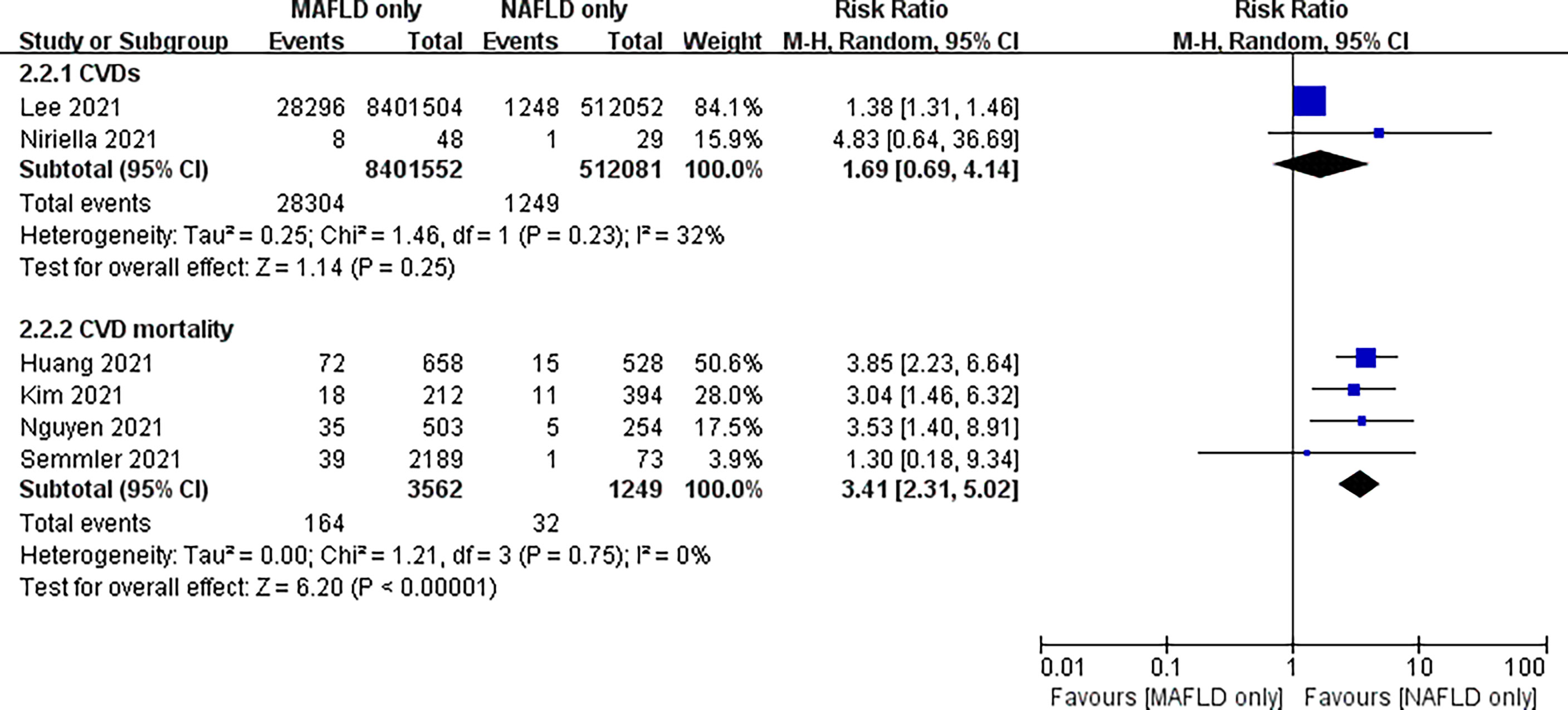
Figure 5 Subgroup analysis of CVD incidence and CVD mortality in the MAFLD-only group and the NAFLD-only group.
Discussion
A total of 10 studies were included in this study, all of which were cohort studies. The relative risk of CVD and death from CVD during the follow-up was significantly increased in the MAFLD group compared with the control group. Overall, the incidence of CVD or CVD mortality was 1.95 times higher in the MAFLD group than in the control group. The incidence of CVD and CVD mortality was 2.26 times and 1.57 times higher in the MAFLD group than in the control group, respectively. Our results were similar to previous findings. A 4.6-year cohort study from China showed that MAFLD was associated with a higher risk of CVD (hazard ratio, 1.44; 95% CI 1.15–1.81) (22). In addition, the study found that the cardiovascular mortality rate in the MAFLD-only group was 3.41 times higher than that in the NAFLD-only group; our study found no statistically significant difference in the incidence of CVD between the two groups. We believed that the differences between the MAFLD-only group and the NAFLD-only group might be related to the number of studies included and the sample size. According to the existing data, whether the newly defined MAFLD would lead to a higher cardiovascular risk than NAFLD under the old definition was not clear. We considered that the new diagnostic criteria for the fatty liver disease might be easier to identify its correlation with CVD. Previous studies showed that MAFLD was better at identifying patients with acute CVD events or exacerbations of acute CVD risk than patients with NAFLD (29–31). However, these studies were retrospective studies using a previous database. Further prospective studies are still needed to compare the differences between MAFLD and NAFLD.
The pathogenesis of MAFLD is complex and multifactorial, with currently no clear explanation of how MAFLD affects CVD incidence and CVD mortality. Studies pointed out that MAFLD was associated with CVD. The increased morbidity and all-cause mortality could be attributed to several pathogenic factors, including hormones, nutrition, toxicity of intestinal disorders, insulin resistance, fat, liver inflammation, and gene functions (32–34). Adipose tissue is a highly active endocrine tissue that produces peptide adipocytokines with autocrine, paracrine, and endocrine functions, such as adiponectin and endolipins (35). Adiponectin is the most abundant peptide secreted by adipocytes; it has been found to be secreted by other cells besides endothelial cells, including bone and cardiomyocytes. Reduced adiponectin levels play a critical role in obesity-related pathology, such as insulin resistance, type 2 diabetes, and CVD (36). Studies have shown that adiponectin has insulin sensitization, anti-atherosclerosis, and anti-inflammatory properties (37–39). Insulin resistance and inflammation are also risk factors for MAFLD and CVD. Despite no evidence that adiponectin affects the incidence of CVD or CVD mortality in patients with MAFLD, we hypothesized that decreased adiponectin levels might increase cardiovascular risk in patients with MAFLD. In addition, elevated lipid levels have been shown to be associated with atherosclerotic disease and coronary artery disease (40). However, Ismaiel et al. showed no significant association between serum adiponectin and enadiponectin levels in patients with MAFLD and controls; although E/A was significantly associated with adiponectin in univariate analysis, this association weakened after multivariable linear regression (35). Considering the effects of adiponectin and enadiponectin on CVD, further studies are needed to confirm their effects on cardiovascular risk in patients with MAFLD.
Recent findings pointed out cardiac contraction, subclinical contraction dysfunction, and increased risk of diastolic dysfunction in patients with MAFLD. Moreover, a low level of ammonia acyl tyrosine was related to lower left ventricular systolic function; even after adjusting confounding factors, lower lysophosphatidylcholine (LPC) (speech/0-0) levels were also associated with diastolic dysfunction (41). This finding was consistent with previous studies assessing cardiac systolic, subclinical systolic, and diastolic function in patients with NAFLD (42, 43). Given the increased risk of CVD in patients with MAFLD, it is necessary to evaluate the association of MAFLD with CVD incidence and mortality against new diagnostic criteria to prevent further cardiovascular complications and an increase in CVD-related morbidity. This new definition is a major breakthrough in the clinical practice of fatty liver disease. More scientific research evidence is still needed to promote the use of this definition in the future.
The results of our study were similar to previous findings; however, this study still had some deficiencies. First, we included only the English literature, omitting many non-English reports. Second, the included literature lacked specific data for analysis, such as sex, age, and previous underlying diseases. Hence, it was impossible to conduct subgroup analysis to exclude the influence of these confounding factors on the study results.
Conclusions
MAFLD significantly increases the risk of CVD and CVD-related mortality. Furthermore, based on the available data, patients diagnosed with MAFLD alone had higher cardiovascular mortality than those diagnosed with NAFLD alone. The association between MAFLD and CVD appears to be more likely under the new diagnostic criteria. However, further studies are needed to demonstrate different effects of the newly defined MAFLD on CVD compared with previous NAFLD.
Author contributions
HL and MW conceived the study and designed the analysis. YC, CC and QW performed statistical analysis. ZF, JT and WW wrote the first draft of the manuscript. MZ, XH and XZ participate in revision the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Hangzhou Science and Technology Bureau fund (No. 20191203B96, No. 20191203B105, and No. 20191231Y039); the Youth Fund of Zhejiang Academy of Medical Sciences (No. 2019Y009); the Medical and Technology Project of Zhejiang Province (No. 2020362651 and No. 2021KY890); the Clinical Research Fund of Zhejiang Medical Association (No. 2020ZYC-A13); the Hangzhou Health and Family Planning Technology Plan Key Projects (No. 2017ZD02); the Hangzhou Medical and Health Technology Project (No. 0020290592); and the Zhejiang Traditional Chinese Medicine Scientific Research Fund Project (No. 2022ZB280).
Acknowledgments
The work was supported by the Key Medical Disciplines of Hangzhou.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (2016) 64:73–84. doi: 10.1002/hep.28431
2. Huang DQ, El-Serag HB, Loomba R. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol (2021) 18:223–38. doi: 10.1038/s41575-020-00381-6
3. Patel SS, Siddiqui MS. Current and emerging therapies for non-alcoholic fatty liver disease. Drugs (2019) 79:75–84. doi: 10.1007/s40265-018-1040-1
4. Targher G, Byrne CD, Lonardo A, et al. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol (2016) 65:589–600. doi: 10.1016/j.jhep.2016.05.013
5. Morrison AE, Zaccardi F, Khunti K, Davies MJ. Causality between non-alcoholic fatty liver disease and risk of cardiovascular disease and type 2 diabetes: A meta-analysis with bias analysis. Liver Int (2019) 39:557–67. doi: 10.1111/liv.13994
6. Ismaiel A, Popa SL, Dumitrascu DL. Acute coronary syndromes and nonalcoholic fatty liver disease: “Un affaire de coeur”. Can J Gastroenterol Hepatol (2020) 2020:8825615. doi: 10.1155/2020/8825615
7. Kovalic AJ, Satapathy SK. The role of nonalcoholic fatty liver disease on cardiovascular manifestations and outcomes. Clin Liver Dis (2018) 22:141–74. doi: 10.1016/j.cld.2017.08.011
8. Liu Y, Zhong GC, Tan HY, Hao FB, Hu JJ. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: A meta-analysis. Sci Rep (2019) 9:11124. doi: 10.1038/s41598-019-47687-3
9. Dongiovanni P, Paolini E, Corsini A, Sirtori CR, Ruscica M. Nonalcoholic fatty liver disease or metabolic dysfunction-associated fatty liver disease diagnoses and cardiovascular diseases: From epidemiology to drug approaches. Eur J Clin Invest (2021) 51:e13519. doi: 10.1111/eci.13519
10. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
11. Eslam M, Sanyal AJ, George J, International Consensus Panel. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology (2020) 158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312
12. Gofton C, George J. Updates in fatty liver disease: Pathophysiology, diagnosis and management. Aust J Gen Pract (2021) 50(10):702–7. doi: 10.31128/AJGP-05-21-5974
13. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol (2019) 4:389–98. doi: 10.1016/S2468-1253(19)30039-1
14. Niriella MA, Ediriweera DS, Kasturiratne A, De Silva ST, Dassanayaka AS, De Silva AP, et al. Outcomes of NAFLD and MAFLD: Results from a community-based, prospective cohort study. PloS One (2021) 16:e0245762. doi: 10.1371/journal.pone.0245762
15. Lee H, Lee YH, Kim SU, Kim HC. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: A nationwide cohort study. Clin Gastroenterol Hepatol (2021) 19:2138–2147.e10. doi: 10.1016/j.cgh.2020.12.022
16. Wei J, Liu S, Wang X, Li B, Qiao L, Wang Y, et al. Efficacy and safety of shexiang baoxin pill for coronary heart disease after percutaneous coronary intervention: A systematic review and meta-analysis. Evid Based Complement Alternat Med (2021) 2021:2672516. doi: 10.1155/2021/2672516
17. Roth GA, Mensah GA, Fuster V. The global burden of cardiovascular diseases and risks: A compass for global action. J Am Coll Cardiol (2020) 76:2980–1. doi: 10.1016/j.jacc.2020.11.021
18. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: Update from the GBD 2019 study. J Am Coll Cardiol (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
19. Shao C, Wang J, Tian J, Tang YD. Coronary artery disease: From mechanism to clinical practice. Adv Exp Med Biol (2020) 1177:1–36. doi: 10.1007/978-981-15-2517-9_1
20. Olvera Lopez E, Ballard BD, Jan A. Cardiovascular disease. 2021. Treasure Island (FL: StatPearls Publishing (2022).
21. Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol (2021) 19:2172–2181.e6. doi: 10.1016/j.cgh.2021.05.029
22. Liang Y, Chen H, Liu Y, Hou X, Wei L, Bao Y, et al. Association of MAFLD with diabetes, chronic kidney disease, and cardiovascular disease: A 4.6-year cohort study in China. J Clin Endocrinol Metab (2022) 107:88–97. doi: 10.1210/clinem/dgab641
23. Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the united states. J Hepatol (2021) 75:1284–91. doi: 10.1016/j.jhep.2021.07.035
24. Yoneda M, Yamamoto T, Honda Y, Imajo K, Ogawa Y, Kessoku T, et al. Risk of cardiovascular disease in patients with fatty liver disease as defined from the metabolic dysfunction associated fatty liver disease or nonalcoholic fatty liver disease point of view: A retrospective nationwide claims database study in Japan. J Gastroenterol (2021) 56:1022–32. doi: 10.1007/s00535-021-01828-6
25. Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: Which has closer association with all-cause and cause-specific mortality?-results from NHANES III. Front Med (Lausanne) (2021) 8:693507. doi: 10.3389/fmed.2021.693507
26. Liu Z, Suo C, Shi O, Lin C, Zhao R, Yuan H, et al. The health impact of MAFLD, a novel disease cluster of NAFLD, is amplified by the integrated effect of fatty liver disease-related genetic variants. Clin Gastroenterol Hepatol (2020) 20):S1542–3565. doi: 10.1016/j.cgh.2020.12.033
27. Semmler G, Wernly S, Bachmayer S, Leitner I, Wernly B, Egger M, et al. Metabolic dysfunction-associated fatty liver disease (MAFLD)-rather a bystander than a driver of mortality. J Clin Endocrinol Metab (2021) 106:2670–7. doi: 10.1210/clinem/dgab339
28. Liu HH, Cao YX, Jin JL, Guo YL, Zhu CG, Wu NQ, et al. Metabolic-associated fatty liver disease and major adverse cardiac events in patients with chronic coronary syndrome: a matched case-control study. Hepatol Int (2021) 15:1337–46. doi: 10.1007/s12072-021-10252-0
29. Kawaguchi T, Tsutsumi T, Nakano D, Torimura T. MAFLD: Renovation of clinical practice and disease awareness of fatty liver. Hepatol Res (2021) 52(5):422–432. doi: 10.1111/hepr.13706
30. Guerreiro GTS, Longo L, Fonseca MA, de Souza VEG, Alvares-da-Silva MR. Does the risk of cardiovascular events differ between biopsy-proven NAFLD and MAFLD? Hepatol Int (2021) 15:380–91. doi: 10.1007/s12072-021-10157-y
31. Tsutsumi T, Eslam M, Kawaguchi T, Yamamura S, Kawaguchi A, Nakano D, et al. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: Generalized estimating equation approach. Hepatol Res (2021) 51:1115–28. doi: 10.1111/hepr.13685
32. Carr RM, Oranu A, Khungar V. Nonalcoholic fatty liver disease: Pathophysiology and management. Gastroenterol Clin North Am (2016) 45:639–52. doi: 10.1016/j.gtc.2016.07.003
33. Ismaiel A, Dumitrascu DL. Genetic predisposition in metabolic-dysfunction-associated fatty liver disease and cardiovascular outcomes-systematic review. Eur J Clin Invest (2020) 50:e13331. doi: 10.1111/eci.13331
34. Ismaiel A, Dumitraşcu DL. Cardiovascular risk in fatty liver disease: The liver-heart axis-literature review. Front Med (Lausanne) (2019) 6:202. doi: 10.3389/fmed.2019.00202
35. Ismaiel A, Spinu M, Budisan L, Leucuta DC, Popa SL, Chis BA, et al. Relationship between adipokines and cardiovascular ultrasound parameters in metabolic-dysfunction-associated fatty liver disease. J Clin Med (2021) 10(21):5194. doi: 10.3390/jcm10215194
36. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci (2017) 18:1321. doi: 10.3390/ijms18061321
37. Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem (2002) 277:37487–91. doi: 10.1074/jbc.M206083200
38. Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein e-deficient mice. Circulation (2002) 106:2767–70. doi: 10.1161/01.CIR.0000042707.50032.19
39. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med (2001) 7:941–6. doi: 10.1038/90984
40. Duman H, Özyıldız AG, Bahçeci İ, Duman H, Uslu A, Ergül E. Serum visfatin level is associated with complexity of coronary artery disease in patients with stable angina pectoris. Ther Adv Cardiovasc Dis (2019) 13:1753944719880448. doi: 10.1177/1753944719880448
41. Ismaiel A, Spinu M, Socaciu C, Budisan L, Leucuta DC, Popa SL, et al. Metabolic biomarkers related to cardiac dysfunction in metabolic-dysfunction-associated fatty liver disease: A cross-sectional analysis. Nutr Diabetes (2022) 12:4. doi: 10.1038/s41387-022-00182-7
42. Wijarnpreecha K, Lou S, Panjawatanan P, Cheungpasitporn W, Pungpapong S, Lukens FJ, et al. Association between diastolic cardiac dysfunction and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Dig Liver Dis (2018) 50:1166–75. doi: 10.1016/j.dld.2018.09.004
Keywords: cardiovascular disease (CVD), MAFLD (metabolic associated fatty liver disease), myocardial infarction, stroke, transient ischemic attack
Citation: Wen W, Li H, Wang C, Chen C, Tang J, Zhou M, Hong X, Cheng Y, Wu Q, Zhang X, Feng Z and Wang M (2022) Metabolic dysfunction-associated fatty liver disease and cardiovascular disease: A meta-analysis. Front. Endocrinol. 13:934225. doi: 10.3389/fendo.2022.934225
Received: 09 May 2022; Accepted: 26 August 2022;
Published: 16 September 2022.
Edited by:
Rabia Johnson, South African Medical Research Council, South AfricaReviewed by:
Robert G. Gish, Loma Linda University, United StatesJuan Pablo Roblero, Clinical Hospital of the University of Chile, Chile
Hieu Trung Le, 108 Military Central Hospital, Vietnam
Copyright © 2022 Wen, Li, Wang, Chen, Tang, Zhou, Hong, Cheng, Wu, Zhang, Feng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanhui Feng, h9450203@126.com; Mingwei Wang, wmw990556@163.com
†These authors have contributed equally to this work
 Wen Wen1†
Wen Wen1† Chunyi Wang
Chunyi Wang Mengyun Zhou
Mengyun Zhou Yongran Cheng
Yongran Cheng Zhanhui Feng
Zhanhui Feng Mingwei Wang
Mingwei Wang