Value of FT3/FT4 Ratio in Prognosis of Patients With Heart Failure: A Propensity-Matched Study
- Department of Cardiology, Shengjing Hospital of China Medical University, Shenyang, China
Aims: Abnormal thyroid hormone secretions can alter the manifestation and prognosis of cardiovascular disease. To assess the effect of the free triiodothyronine (FT3)/free thyroxine (FT4) ratio on the prognosis of patients with heart failure (HF), we performed a propensity-matched study on patients with well-balanced baseline characteristics.
Methods: Overall, 8,887 patients with HF were divided into two groups according to the FT3/FT4 ratio. Propensity scores were calculated from each patient. A cohort comprising 2,164 pairs with high or low ratios and with 34 well-balanced baseline characteristics was then assembled. The endpoints were Cardiovascular (CV) mortality and all-cause mortality. The correlation between FT3/FT4 ratio and prognosis was assessed using matched Cox regression analyses. The mean follow-up was 3.3 years.
Results: In the full pre-match cohort, 3,710 (41.7%) patients died, with 2,581 (29.0%) cases of CV mortality. In the matched-pair cohort, all-cause mortality occurred in 923 (1,238/10,000 person-years of follow-up) patients with a high ratio and 1,036 (1,484/10,000 person-years) patients with a low ratio, resulting in a matched HR of 0.841 (95% CI: 0.769–0.919; P < 0.001). For CV mortality, the result was 638 (856/10,000 person-years) and 714 (1,023/10,000 person-years) patients, respectively, resulting in a matched HR of 0.844 (95% CI: 0.759–0.940; P < 0.001). Subgroup analysis revealed that a low FT3/FT4 ratio had a greater predictive value for all-cause and CV mortality in elderly or male patients and in patients with coronary artery disease (CAD), hypertension, diabetes mellitus, HFmrEF, or HFpEF.
Conclusions: A low FT3/FT4 ratio is valuable for predicting CV mortality and all-cause mortality in patients with HF.
Introduction
Heart failure (HF) is a serious or advanced stage of any heart disease that has remained a major public health threat despite advances in medical therapy (1, 2). The prevalence of HF in developed countries ranges from 1.5 to 2.0% and increases significantly with age, with a reported prevalence of ≥10% in patients older than 70 years (3). Moreover, patients with HF have consistently been associated with a poor quality of life, reporting an in-hospital and 5-year mortality of up to 4.1% (4) and 50% (1, 2), respectively. Therefore, it is important to establish an individualized approach to improve the symptoms and prognosis of patients with HF. In particular, biomarkers can mirror the physical functions and affect patient outcomes, in addition to predicting the prognosis (5). Previous studies have even shown that biomarkers and relative mechanisms-guided management would be helpful in the prognostication, diagnosis, and treatment of patients with HF (6, 7).
Free triiodothyronine (FT3) and free thyroxine (FT4) are two major thyroid hormones that affect the physiological and pathological processes of the cardiovascular system (8–10). Studies show that low T3 syndrome has been associated with poor prognosis in patients with HF (11, 12). FT3/FT4 ratio has also been significantly correlated with adverse outcomes in patients with acute coronary syndrome (8, 9). Despite these findings, no recent literature on the effect of FT3/FT4 ratio on the prognosis of patients with HF has been found.
Traditional multivariable risk adjustment models based on regression are limited by model assumptions, which may not always be appropriate and can become a concern for residual bias and procedural transparency (13). However, propensity-matched cohort can be used to assemble two groups of patients with balanced baseline covariates (14, 15). Thus, in our present large-scale retrospective cohort study, we deduced that the FT3/FT4 ratio would be a significant biomarker for the prediction of long-term outcome in a propensity-matched cohort of patients with HF.
Materials and Methods
Study Population
Retrospective clinical data were collected from patients with HF hospitalized in the Department of Cardiology, Shengjing Hospital of China Medical University, Shenyang, China between 2013 and 2018. HF was diagnosed based on symptoms and signs, elevated levels of natriuretic peptides and at least one additional criterion of relevant structural heart disease (left atrial enlargement or left ventricular hypertrophy) or diastolic dysfunction (16). In accordance with the cardiac function classification published by the New York Heart Association (NYHA), heart function was divided into four levels (II–IV). Furthermore, HF with reduced ejection fraction (HFrEF) was defined as having a left ventricular ejection fraction (LVEF) of <40%, HF with mid-range LVEF (HFmrEF) was defined as having an LVEF of ≥40% but <50%, and HF with preserved LVEF (HFpEF) was defined as having an LVEF ≥50%. Patients displaying evidence of acute myocardial infarction, severe hepatic or renal failure, severe anemia, severe infection, thyroid disease (hyperthyroidism or hypothyroidism), or malignancy were excluded. This study was approved by the Shengjing Hospital of China Medical University Ethics Committee and was carried out in accordance with the principles of the Declaration of Helsinki. The ethics approval number is 2019PS594K.
Patients
Our cohort retrospectively included 8,887 patients with HF hospitalized from January 2013 to December 2018. The investigators extracted their corresponding comprehensive clinical data from the electronic medical records. Obtained variables included patient demographics, past cardiac and non-cardiac history, physical examination results, laboratory test results, and echocardiography. All laboratory tests of the fasting peripheral venous blood samples were also taken on the day of admission or the morning after admission. LVEF was determined by echocardiography using the biplane Simpson method within 3 days of admission. In December 2020, efforts were made to determine the nature of death in each case, patients' survival status were also investigated using the population death information registration management system of the Disease Control and Prevention Center of Liaoning Province, wherein cardiac and non-cardiac death was determined in accordance with the International Classification of Diseases (ICD) code of death diagnosis. When information was not available in the system, data were obtained from the medical records, patients' physicians, or patients' relatives via telephone.
Statistical Analysis
To avoid potential confounders and selection biases, we utilized propensity score matching. For the unmatched and matched populations, differences in the baseline characteristics were tested with chi-square and t-tests for categorical and continuous variables, respectively. The optimal cutoff value for the FT3/FT4 ratio was determined with the receiver operating characteristic (ROC) curve. The propensity score (PS) for FT3/FT4 category was separately calculated by a logistic regression model to reduce the selection bias. The clinically relevant variables, which had significant difference between high FT3/FT4 ratio and low FT3/FT4 ratio groups at baseline were included as covariates (Table 1). High FT3/FT4 ratio and low FT3/FT4 ratio were then matched 1:1 using the nearest neighbor method with a caliper of <0.01 without any replacement. The ability of the matching to balance baseline characteristics in high vs. low ratios was assessed using absolute standard differences and a quartile, reporting a non-significant value of <10%. The absolute standardized differences before and after matching were shown as Love plots. Primary outcomes of this study included 8-year all-cause mortality and 8-year CV mortality. Cox proportional hazards models were also used in overall cohort adjustment and matched population to estimate the association between the FT3/FT4 ratio and outcomes. Results were presented with their hazard ratio (HR) and corresponding 95% confidence interval (CI), and survival estimates were visualized using the Kaplan–Meier method. Interaction analyses were further conducted with consideration to the age, gender, CAD, hypertension, diabetes mellitus, stroke, atrial fibrillation, previous myocardial infarction, and LVEF in the matching population. All statistical analyses were performed using the R software version 3.6.1, and a two-tailed P-value of <0.05 was considered to be statistically significant.
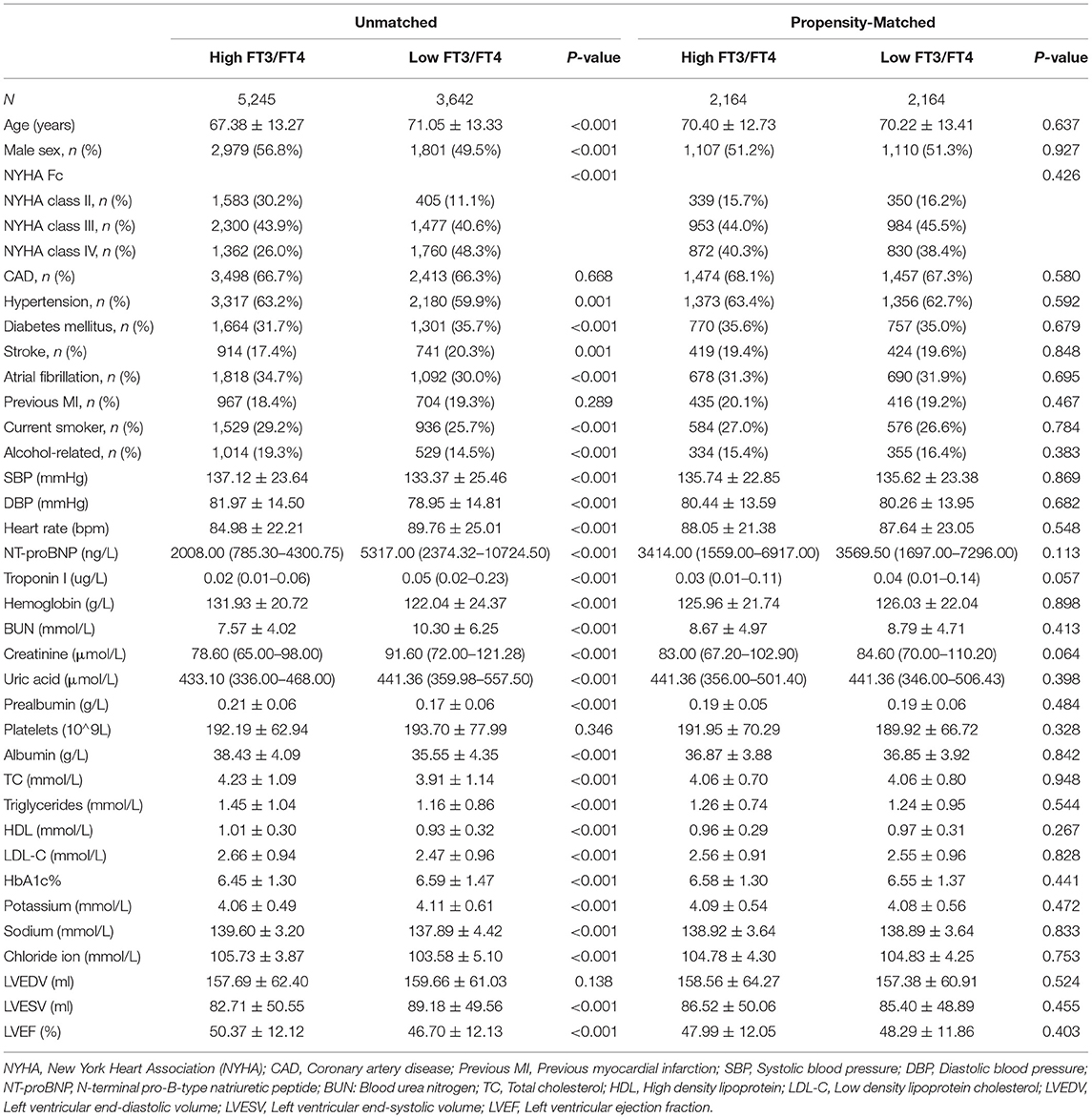
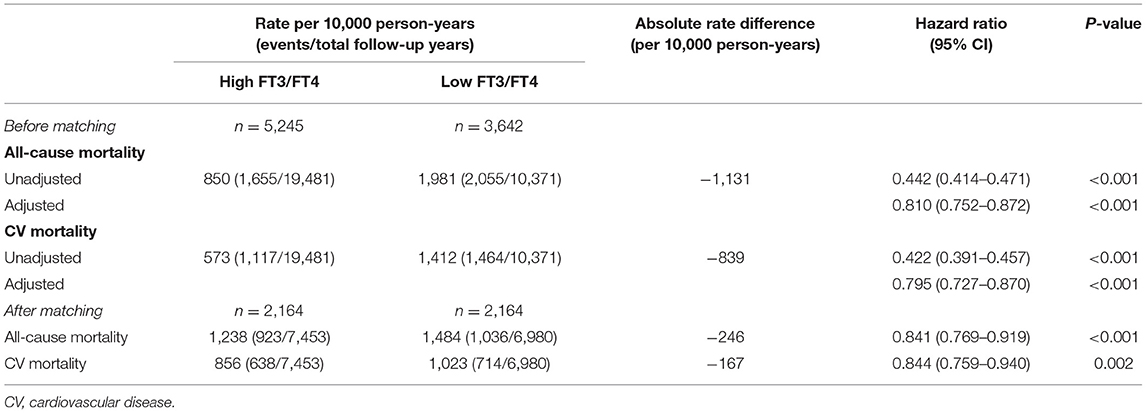
Table 1. Baseline characteristics of patients in the high and low FT3/FT4 ratio groups before and after propensity matching.
Results
Baseline Characteristics
A total of 8,887 patients met the inclusion criteria. Patients in the cohort had a mean age of 69 (± 13) years, and 53.8% were male. The median follow-up time was 3.3 years (2–8 years).The optimal cutoff value for FT3/FT4 is 0.233, which was determined with the receiver operating characteristic (ROC) curve, with a area under the ROC curve (AUC) of 0.668. According to this cutoff value, FT3/FT4 is divided into two groups: the high FT3/FT4 (0.233~0.563) and low FT3/FT4 ratio group (0.085~0.233). The propensity score for the FT3/FT4 category was separately calculated using a logistic regression model, and the clinically relevant variables listed in Table 1 were used as covariates. Prior to matching, patients in the high FT3/FT4 ratio group were found to be younger, more likely to be male, had a more severe NYHA heart function, and had lower NT-proBNP levels than those in the low FT3/FT4 ratio group (Table 1, Figure 1). Other significant imbalances in baseline characteristics before matching and the balances after matching are displayed in Table 1, Figure 1. After matching, absolute standardized differences for all measured covariates were <10% (usually 5%), indicating significant covariate balance between the two groups (Figure 1).

Figure 1. Love plots for absolute standardized differences for baseline covariates of patients between high and low FT3/FT4 ratio group, before and after propensity score matching.
FT3/FT4 Ratio and Mortality
Overall, in the full pre-match cohort of 8,887 patients, 3,710 (41.7%) patients died, with 2,581 (29.0%) cases of CV mortality, during a median follow-up of 3.2 years. All-cause mortality occurred in 1,655 (rate, 850/10,000 person-years of follow-up) patients with a high FT3/FT4 ratio and 2,055 (rate, 1,981/10,000 person-years) patients with a low ratio, resulting in an unadjusted HR of 0.442 (95% CI: 0.414–0.471; P < 0.001) and an adjusted HR of 0.810 (95% CI: 0.752–0.872; P < 0.001) (Table 2). Meanwhile, CV mortality occurred in 1,117 (rate, 573/10,000 person-years) patients with a high FT3/FT4 ratio and 1,464 (rate, 1,412/10,000 person-years) patients with a low ratio, resulting in an unadjusted HR of 0.422 (95% CI:0.391–0.457; P < 0.001) and an adjusted HR of 0.795 (95% CI: 0.727–0.870; P < 0.001) (Table 2).
In the matched-pair cohort of 2,164 patients, all-cause mortality occurred in 923 (rate, 1,238/10,000 person-years) patients with a high FT3/FT4 ratio and 1,036 (rate, 1,484/10,000 person-years) patients with a low ratio, resulting in a matched HR of 0.841 (95% CI: 0.769–0.919; P < 0.001) (Figure 2A, Table 2). For CV mortality, this was observed in 638 (rate, 856/10,000 person-years) patients with a high FT3/FT4 ratio and 714 (rate, 1,023/10,000 person-years) patients with a low ratio, resulting in a matched HR of 0.844 (95% CI: 0.759–0.940; P < 0.001) (Figure 2B, Table 2).
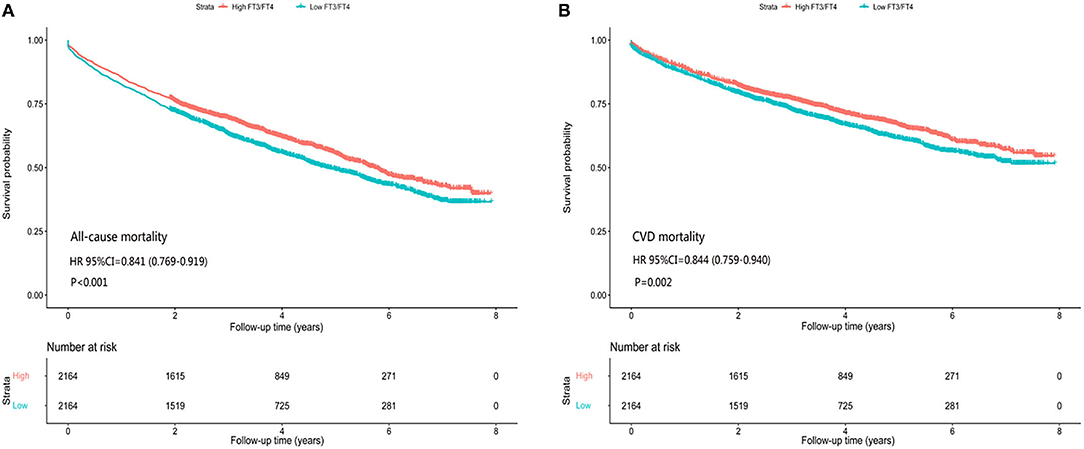
Figure 2. Association of high and low FT3/FT4 ratio with all-cause/CV mortality in subgroups of propensity score-matched patients, (A) all-cause mortality, (B) CV mortality.
Findings from our sensitivity analysis indicate that the covariate (FT3/FT4), which is a predictor of mortality in patients with HF, may potentially explain the association between HF and mortality.
Correlations between the FT3/FT4 ratio and all-cause/CV mortality among various subgroups are displayed in Figure 3. Generally, a low FT3/FT4 ratio had a greater predictive value for all-cause mortality and CV mortality in elderly or male patients and in patients with CAD, hypertension, diabetes mellitus, HFmrEF, or HFpEF.

Figure 3. Association of high and low FT3/FT4 ratio with all-cause/CV mortality in subgroups of propensity score-matched patients, (A) all-cause mortality, (B) CV mortality.
Discussion
In our retrospective cohort of 8,887 patients, 3,710 (41.7%) patients died, with 2,581 (29.0%) cases of CV mortality, during a median follow-up of 3.2 years. In the matched-pair cohort of 2,164 pairs of patients with high and low FT3/FT4 ratios and with 34 balanced baseline characteristics, we found that all-cause and CV mortality in patients with HF could be predicted independently using a low FT3/FT4 ratio. Specifically, the hazard ratio of long-term all-cause mortality for patients with a high FT3/FT4 ratio was 0.841 times less than that in patients with a low FT3/FT4 ratio. This was similarly seen in CV mortality, with a value of 0.844.
Baseline analysis showed that a lower FT3/FT4 ratio was associated with worse heart function and clinical characteristics, including advanced age; higher rates of diabetes and stroke; increased troponin I and NT-probNP; and decreased hemoglobin, albumin, and sodium; all of which may be related to poor prognosis in HF. Numerous studies have also shown that reduced FT3 was associated with increased cardiovascular morbidity and mortality (including HF) (10, 12), and high FT4 level within normal thyroid function has been correlated with HF and sudden cardiac death (17, 18). In particular, Kannan et al. demonstrated that higher FT4 and lower total triiodothyronine were associated with an increased risk of the composite end point for left ventricular assist device implantation, heart transplantation, or all-cause mortality (19). Furthermore, previous studies have indicated that a decreased FT3/FT4 ratio is associated with an increased risk of long-term all-cause mortality, cardiovascular mortality, and major adverse cardiovascular events in patients with CAD (9, 20).
The cardiovascular system is an important target organ of thyroid hormones, as their main effect on this system increases the heart rate, enhances myocardial contractility, reduces circulation resistance, and relieves cardiac afterload (21, 22). Thyroid hormone abnormalities, such as thyroid function hyperfunction, hypothyroidism, and low T3 syndrome, can influence the clinical manifestations and outcomes of cardiovascular disease (11). Subclinical hyperthyroidism, subclinical hypothyroidism, and low T3 syndrome are associated with high risk of atrial fibrillation and increased mortality of patients with cardiac disease (23, 24). Even a minor alteration in thyroid hormone concentration will affect the pathological and physiological process of the cardiovascular system (25, 26). Among these thyroid hormones, FT3 and FT4 are usually more sensitive and clinically relevant than T3 and T4, given that former are the physiologically active forms of the latter (27).
Previous studies have demonstrated that the FT3/FT4 ratio could reflect deiodinase activity (28), so the decline of this ratio may reflect the reduced transformation from T4 to T3 in the peripheral blood (27, 29). Therefore, the possible mechanisms of the influence of FT3/FT4 ratio on HF prognosis can be as follows. First, insufficient FT3 transformation leads to more obvious oxidative stress damage of the endoplasmic reticulum and impaired utilization of ATP by cardiomyocytes, resulting in an impaired systolic function and development of HF (30). Second, there are specific T3 receptors in the myocardium, and the decrease of FT3 may lower myocardial contractility and increase susceptibility to arrhythmia, possibly resulting in death in patients with HF (12). Third, low levels of FT3 are related to the increase of right atrial pressure, pulmonary artery pressure, and pulmonary capillary wedge pressure, as well as the decrease of ejection fraction and cardiac index, which results in myocardial fibrosis, ventricular remodeling, and myocardial perfusion and metabolism abnormalities (30). Furthermore, the FT3/FT4 ratio may be an important predictor of metabolic disease (31) and acute myocardial infarction (20).
Given the effect of thyroid hormones on the prognosis of heart failure, some scholars believe that replacement therapy, regulation of deiodinase activity, and heart-specific thyroid receptor agonists are potential treatments for HF (32). However, the potential benefits of thyroid hormone supplementation for these patients should be weighed against the risks of overtreatment. Moreover, the tolerability and safety of the aforementioned treatment regimens need further studies to confirm their viability.
Despite the large sample of the present study, a few limitations were noted. First, although we eliminated the influence of confounding factors and selection bias using propensity-matched scoring, we consequently missed a large portion of real-world data. Second, FT3 and FT4 were measured at baseline without dynamic monitoring, which may have limited the accuracy of the results. Lastly, we failed to mention and analyze the drug use of the included patients. This is important since iodized contrast agents, including amiodarone and glucocorticoids, might have affected thyroid function.
In conclusion, this propensity-matched study revealed that a low FT3/FT4 ratio had a greater predictive value for all-cause mortality and CV mortality, especially in elderly and male patients and in patients with CAD, hypertension, diabetes mellitus, HFmrEF, or HFpEF.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Shengjing Hospital of China Medical University Ethics Committee and was carried out in accordance with the principles of the Declaration of Helsinki. The ethics approval number is 2019PS594K. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CW and ZS conceived and designed the study and wrote the paper. CW, SH, and YL extracted and sorted clinical data. ZL and FT analyzed the data. All authors contributed to the article and approved the submitted version.
Funding
The present study was financially supported by a grant from the Science and Technology Program of Liaoning Province (No. 2018225003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The statistical analysis contributions of Hao Sun at the 1st Hospital of China Medical University is gratefully acknowledged.
References
1. Yancy CW, Jessup M, Bozkurt B, Jessup M, Bozkurt B, Butler J, et al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart failure society of America. J Am Coll Cardiol. (2017) 70:776–803. doi: 10.1161/CIR.0000000000000509
2. Taylor CJ, Ordóñez-Mena JM, Roalfe AK, Lay-Flurrie S, Jones NR, Marshall, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000-2017: population based cohort study. BMJ. (2019) 364:l223. doi: 10.1136/bmj.l223
3. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. (2007) 93:1137–46. doi: 10.1136/hrt.2003.025270
4. Zhang Y, Zhang J, Butler J, Yang XM, Xie PY, Guo DS, et al. Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in China: results from the China heart failure (China-HF) registry. J Card Fail. (2017) 23:868–75. doi: 10.1016/j.cardfail.2017.09.014
5. Wang CH, Han S, Tong F, Li Y, Li ZC, Sun ZJ. Predictive value of the serum cystatin C/Prealbumin ratio in combination with NT-proBNP levels for long-term prognosis in chronic heart failure patients: a retrospective cohort study. Front Cardiovasc Med. (2021) 8:684919. doi: 10.3389/fcvm.2021.684919
6. Sarhene M, Wang Y, Wei J, Huang Y, Li M, Li L, et al. Biomarkers in heart failure: the past, current and future. Heart Fail Rev. (2019) 24:867–903. doi: 10.1007/s10741-019-09807-z
7. Dungen HD, Petroni R, Correale M, Coiro S, Monitillo F, Triggiani M, et al. A new educational program in heart failure drug development: the Brescia international master program. J Cardiovasc Med. (2018) 19:411–21. doi: 10.2459/JCM.0000000000000669
8. Smith SJ, Bos G, Gerbrandy J, Docter R, Visser TJ, Hennemann G, et al. Lowering of serum 3,3′,5-triiodothyronine thyroxine ratio in patients with myocardial infarction; relationship with extent of tissue injury. Eur J Clin Investig. (1978) 8:99–102. doi: 10.1111/j.1365-2362.1978.tb00819.x
9. Yuan DH, Zhang C, Jia SD, Liu Y, Jiang, Xu LJ, et al. Predictive value of free triiodothyronine (FT3) to free thyroxine (FT4) ratio in long-term outcomes of euthyroid patients with three-vessel coronary artery disease. Nutr Metab Cardiovasc Dis. (2021) 31:579–86. doi: 10.1016/j.numecd.2020.10.011
10. Razvi S, Jabbar A, Pingitore A, Danzi S, Biondi B, Klein I, et al. Thyroid hormones and cardiovascular function and diseases. J Am Coll Cardiol. (2018) 71:1781–96. doi: 10.1016/j.jacc.2018.02.045
11. Sato Y, Yoshihisa A, Kimishima Y, Kiko T, Kanno Y, Yokokawa T, et al. Low T3 syndrome is associated with high mortality in hospitalized patients with Heart failure. J Card Fail. (2019) 25:195–203. doi: 10.1016/j.cardfail.2019.01.007
12. Okayama D, Minami Y, Kataoka S, Shiga T, Hagiwara N. Thyroid function on admission and outcome in patients hospitalized for acute decompensated heart failure. J Cardiol. (2015) 66:205–11. doi: 10.1016/j.jjcc.2015.04.006
13. Fitzmaurice G. Confounding: regression adjustment. Nutrition. (2006) 22:581–3. doi: 10.1016/j.nut.2006.02.004
14. Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. (2007) 99:393–8. doi: 10.1016/j.amjcard.2006.08.042
15. Ahmed A, Zannad F, Love TE, Tallaj J, Gheorghiade M, Ekundayo OJ, et al. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. (2007) 28:1334–43. doi: 10.1093/eurheartj/ehm091
16. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis treatment of acute chronic heart failure: the Task Force for the diagnosis treatment of acute chronic heart failure of the European Society of Cardiology (ESC). developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2016) 18:891–975. doi: 10.1002/ejhf.592
17. Cappola AR, Arnold AM, Wulczyn K, Carlson M, Robbins J, Psaty BM. Thyroid function in the euthyroid range and adverse outcomes in older adults. J Clin Endocrinol Metab. (2015) 100:1088–96. doi: 10.1210/jc.2014-3586
18. Chaker L, van den Berg ME, Niemeijer MN, Franco OH, Dehghan A, Hofman A, et al. Thyroid function and sudden cardiac death: a prospective population-based cohort study. Circulation. (2016) 134:713–22. doi: 10.1161/CIRCULATIONAHA.115.020789
19. Kannan L, Shaw PA, Morley MP, Brandimarto J, Fang JC, Sweitzer NK, et al. Thyroid dysfunction in heart failure and cardiovascular outcomes. Circ Heart Fail. (2018) 11:e005266. doi: 10.1161/CIRCHEARTFAILURE.118.005266
20. Yu TT, Tian CY, Song J, He DX, Wu JK, Wen ZY, et al. Value of the fT3/fT4 ratio and its combination with the GRACE risk score in predicting the prognosis in euthyroid patients with acute myocardial infarction undergoing percutaneous coronary intervention: a prospective cohort study. BMC Cardiovasc Disord. (2018) 18:181. doi: 10.1186/s12872-018-0916-z
21. Grais IM, Sowers JR. Thyroid and the heart. Am J Med. (2014) 127:691–8. doi: 10.1016/j.amjmed.2014.03.009
22. Bruere H, Fauchier L, Bernard Brunet A, Pierre B, Simeon E, Babuty D, et al. History of thyroid disorders in relation to clinical outcomes in atrial fibrillation: a cohort study. Am J Med. (2015) 128:30–7. doi: 10.1016/j.amjmed.2014.07.014
23. Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. (2006) 295:1033–41. doi: 10.1001/jama.295.9.1033
24. Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, et al. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation. (2003) 107:708–13. doi: 10.1161/01.CIR.0000048124.64204.3F
25. Iervasi G, Molinaro S, Landi P, Taddei MC, Galli E, Mariani F, et al. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med. (2007) 167:1526–32. doi: 10.1001/archinte.167.14.1526
26. Zhang M, Sara JD, Matsuzawa Y, Gharib H, Bell MR, Gulati R, et al. Clinical outcomes of patients with hypothyroidism undergoing percutaneous coronary intervention. Eur Heart J. (2016) 37:2055–65. doi: 10.1093/eurheartj/ehv737
27. Maia AL, Goemann IM, Meyer ELS, Wajner SM. Deiodinases: the balance of thyroid hormone: type 1 iodothyronine deiodi-nase in human physiology and disease. J Endocrinol. (2011) 209:283–97. doi: 10.1530/JOE-10-0481
28. Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest. (2005) 115:2524–33. doi: 10.1172/JCI25083
29. Itoh S, Yamaba Y, Oda T, Kawagoe K. Serum thyroid hormone, triiodothyronine, thyroxine, and triiodothyronine/thyroxine ratio in patients with fulminant, acute, and chronic hepatitis. Am J Gastroenterol. (1986) 81:444–9.
30. van der Molen AJ, Thomsen HS, Morcos SK. Effect of iodinated contrast media on thyroid function in adults. Eur Radiol. (2004) 14:902–7. doi: 10.1007/s00330-004-2238-z
31. Park SY, Park SE, Jung SW, Jin HS, Park IB, Ahn SV et al. Free triiodothyronine/free thyroxine ratio rather than TSH is more associated with metabolic parameters in healthy euthyroid adult subjects. Clin Endocrinol. (2017) 87:87–96. doi: 10.1111/cen.13345
Keywords: heart failure, long-term, mortality, prognosis, FT3/FT4 ratio, propensity-matched
Citation: Wang C, Han S, Li Y, Tong F, Li Z and Sun Z (2022) Value of FT3/FT4 Ratio in Prognosis of Patients With Heart Failure: A Propensity-Matched Study. Front. Cardiovasc. Med. 9:859608. doi: 10.3389/fcvm.2022.859608
Received: 21 January 2022; Accepted: 21 March 2022;
Published: 12 April 2022.
Edited by:
Tong Liu, Tianjin Medical University, ChinaReviewed by:
Qing Zhang, Sichuan University, ChinaKuo Zhang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Ling Sun, Nanjing Medical University, China
Copyright © 2022 Wang, Han, Li, Tong, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijun Sun, sunzj@sj-hospital.org
 Chuanhe Wang
Chuanhe Wang  Su Han
Su Han Zhijun Sun
Zhijun Sun