Primary Mucoepidermoid Carcinoma of the Breast: A Rare Breast Entity
A B S T R A C T
Objectives: The imaging features of Mucoepidermoid carcinoma (MEC) of the breast are unfamiliar to the breast radiologists due to its rarity. In this paper, we aim to review the literature, do a data analysis and find a common pattern that could potentially give us a better understanding of this entity.
Methods: A literature review was performed with searching keywords “mucoepidermoid carcinoma breast”, without limitations in the dates or the article types, in the PubMed database. Information about the number of cases, age of patients, presenting symptoms, time-interval between the onset of symptoms and time of diagnosis, the diagnostic approach, imaging, the treatment and the outcome have been reviewed and tabulated.
Results: We identified 40 cases. The mean age of the patients was 57. The commonest symptom was a palpable mass, with a duration of a few months, for up to 37years prior to diagnosis and size ranging between 5 to 82mm. On US, the lesions appeared as irregular masses or had cystic benign features. The majority of patients underwent a radical/modified radical mastectomy with a form of axillary lymph node procedure. 15 cases were High-grade, 3 Intermediate-grade and 19 Low-grade MEC. No chemotherapy was administered in most cases and in a mean follow-up time of 30 months, 5 died because of metastasis.
Conclusion: Due to its rarity, the diagnostic and therapeutic approach of MEC in the breast is not well-documented, causing challenges in daily practice, even at experienced Breast Centers. Review of the published cases is essential for multidisciplinary team meetings to plan treatment strategies.
Keywords
Breast Cancer, Mucoepidermoid Carcinoma, Salivary Gland
Introduction
Imaging features of rare breast lesions can be unfamiliar to the breast radiologist due to their scarcity and the diagnostic and therapeutic approach are not well-documented in the literature. Although primarily the diagnosis is on the basis of core biopsy, knowledge of their existence and imaging characteristics can improve radiological interpretation and aid the multidisciplinary team discussion and management approach.
Mucoepidermoid carcinoma (MEC) is a tumor that predominantly arises from the major salivary glands and less commonly from minor salivary glands of the oral and nasal cavity. There are reports of these tumors in other parts of the body, including the esophagus and bronchial tree, thyroid gland, thymus, ear and mandible, pancreas, lacrimal gland and skin adnexa [1].
Breast is a rare location for these tumors and in an extensive review of the literature, going back to the 1970’s, we found that up to date only 40 cases have been reported (herein we publish another one). Because of this, there are no specific guidelines regarding the diagnostic pathway, treatment plan or surgical and oncological approaches. Of the previously published papers, only 5 included radiological imaging of those lesions, 6 provided an unclear description of the imaging findings and the remainder focused on the pathological analysis with no imaging information [2-9]. In this paper, we wanted to present a case of MEC showing the diagnostic difficulties, as that it was misdiagnosed as a simple cyst in the initial presentation, show the radiological imaging and the pathological findings, do a systemic review of the literature, data analysis of all the published cases and find a common pattern of those lesions that could potentially give us a better idea of this entity.
Materials and Methods
I Case Presentation
A 60-year-old Bangladeshi patient was diagnosed with a low-grade MEC during the screening services of our department and was discussed at the multidisciplinary meeting. The patient followed the standard diagnostic pathway for screening ladies, according to the National Breast Screening System (NBSS) and NICE guidelines and had 2D mammograms (MLO and CC views), further mammographic views on the symptomatic side, US examination and an US guided core biopsy.
In general, the patient was asymptomatic, with no previous history of breast problems or family history of breast cancer. Her menopause was at the age of 56. She had never been on hormonal replacement therapy. She has had six children which she breastfed. There is no history of alcohol consumption or smoking. From her medical history, only hypertension, hypercholesterolemia and hay fever were reported. Based on the breast findings and due to the lack of experience, an extensive literature review was performed in an attempt to answer important queries concerning the diagnostic and therapeutic approach of this entity. Written informed consent for publication of the patient's clinical details and clinical images was obtained from the patient and her daughter.
II Literature Review
In the PubMed database, searching keywords were used, such as “mucoepidermoid carcinoma breast”, without limitations in the dates, the language or the article types. The relevant articles and referenced sources were also reviewed for additional papers. Information about the number of cases, age of patients, presenting symptoms, the time-interval between the onset of symptoms and time of diagnosis, the type of diagnostic approach and imaging, the treatment and outcome have been tabulated and reviewed.
Figure 1: Mammograms in 2015. a) MLO b) CC view. In the upper outer of the right breast, two isodensity, well-defined opacities seen (arrow and arrow head). The lesions were graded as M2 in the UK 5 point grading system (equivalent to BIRADS-3).
Results
I Case Presentation
During the breast screening program in 2015, a 60-year old Bangladeshi lady was recalled for further investigation, as two relatively well-defined, isodensity opacities were identified in the mammograms of the right breast, measuring 12 mm and 5 mm (Figure 1). In the subsequent US examination (Figure 2), at 10 o’clock, a thin-walled cystic lesion noted measuring 12x8 mm. No internal vascularity was identified with the use of power Doppler. The lesion was graded as U2 in the 5-point UK classification system (equivalent to BIRADS-3) and was aspirated to dryness with the use of a 21-gauge needle [10]. Τhe content was slightly blood-stained and was sent for cytology, which was reported as C2 (normal cystic content). Additionally, 30 mm apart from the cystic lesion, a 5mm well-defined isoechoic nodule was identified, without significant vascularity in the power Doppler. An inspissated cyst and a papillary lesion were in the differential diagnosis and were graded as U3 in the 5-point UK classification system (equivalent to BIRADS-4a) [10]. A 14-gauge needle core biopsy was performed that showed an infarcted papillary lesion, without atypia. Following this, an US-guided vacuum excision with a 10-gauge needle took place and a marker clip was deployed at the side. The patient returned to routine screening.
Figure 2: US imaging in 2015. At 10 o’clock, a cystic lesion seen, measuring 12x8mm (arrow). No internal vascularity identified with the use of power Doppler or wall-thickening. It was graded as U2 in the UK 5-point grading system (equivalent to BIRADS-3). The fine needle aspiration showed cystic content without evidence of malignancy (C2). 30mm apart from the cystic lesion (arrow head), a well-defined isoechoic nodule also noted, measuring 5x4mm. It did not show significant vascularity in the power Doppler. An inspissated cyst and a papillary lesion were in the differential diagnosis. It was graded as U3 in the UK 5-point grading system) (equivalent to BIRADS-4a). A 14G core biopsy performed that showed an infarcted papillary lesion, which in the immunochemistry showed no atypia (B3) and it was subsequently excised with an US guided vacuum system.
Three years later, in patient's new screening mammograms, at the area where the previously described cystic lesion was (at 10 o’ clock), a well-defined opacity was noted, larger in size compared to 2015 (Figure 3). Tomosynthesis views performed and an US scan followed (Figure 4), that showed a 24x12 mm, relatively well-defined cystic lesion without internal vascularity on power Doppler, with echogenic rim, thick cystic wall and internal septation. A complex cyst was reported and was subjected to a core biopsy, causing a partial collapse of the lesion. The radiological and clinical gradings were M3 U3 P2 (not palpable) in the 5-point UK classification system (equivalent to BIRADS-4) [10].
Figure 3: Mammograms in 2018. a) MLO b) CC view. At the area where the previously described cystic lesion was (arrow), in the upper outer quadrant, again, a well-defined, lobulated lesion seen, larger in size compared to 2015. Also, a marker clip is noted (arrow head), at the area of the papillary lesion that was excised under US guided vacuum procedure. It was graded as M3 (UK 5-point grading system) (equivalent to BIRAD-4a).
The histopathology sections showed cores of breast parenchyma with well-defined areas composed of a mixture of mucinous and epidermoid epithelial cells with microcyst formation. The cytological features were relatively bland. Immunohistochemical stains for myosin showed no evidence of myoepithelial cells surrounding these areas. Staining for p63, CK5/6 and CK7 were positive within the epithelial cells. GATA3 was weakly positive. CK20 and CDX2 were negative. In the differential diagnosis, low-grade mucoepidermoid carcinoma, adenosquamous carcinoma and atypical ductal proliferation with metaplasia were considered and graded as B4.
Figure 4: US examination in 2018: It showed a relatively well-defined cystic lesion with thick cystic-wall and internal septation, echogenic rim, without internal vascularity, 24x12mm in size. A complex cyst was reported and targeted for a core biopsy, causing partial collapse of the lesion. It was graded as U3 in the UK 5-point grading system (equivalent to BIRADS-4a). The sections showed cores of breast parenchyma with well-defined areas composed of a mixture of mucinous and epidermoid epithelial cells with microcyst formation (B4).
After MDM discussion, a wire-guided excision biopsy (Figure 5) was decided upon and performed. The excision contained a 21 mm well-circumscribed lesion composed of cystic spaces set in a sclerotic, hyalinised stroma. The cysts were lined by a mixture of squamous and mucinous epithelium with mild cytological atypia.
Figure 5: Wire localization mammograms (a) MLO, b) CC view) that show a Hawkin’s III wire going through the lesion in the upper outer quadrant of the right breast. The surgical specimen c) shows the lesion situated within the specimen.
Focally the wall of the largest cyst was replaced by chronic inflammatory cell infiltrate and foamy histiocytes, consistent with cyst rupture. Immunohistochemistry showed that the cells within the cystic lesion were positive for AE1/AE3, GATA-3, CK5/6, CK7, P63 and weakly positive for ER. CK20 was negative. Ki-67 proliferation index was low to moderate (Figure 6). The findings confirmed the diagnosis of low-grade mucoepidermoid carcinoma. Adjacent focal intermediate grade Ductal Carcinoma In-Situ (DCIS) was present with morphological appearances similar to that seen in the primary lesion. The histopathological stage was pT2N0.
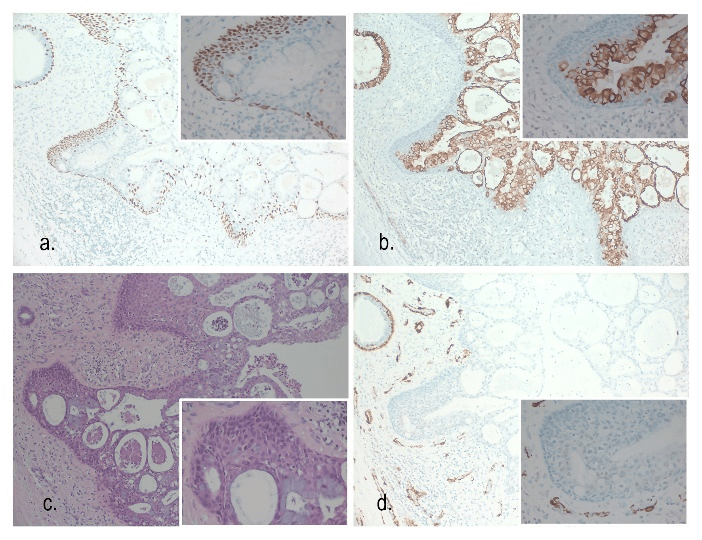
Figure 6: a) p63 100x – Strong positive staining in the squamoid component with negative staining in the luminal mucinous component. Positive staining is apparent in the myoepithelial layer of the normal breast duct (top left). p63 400x (inset). b) CK7 100x. CK7 highlights the luminal mucinous tumour cells but is absent in the squamoid component. A normal breast duct is seen top left showing positive staining. CK7 400x (inset). c) H&E 100x. H&E stain showing tumour with mucinous component (pale blue), squamous and basaloid cells (eosinophilic). A benign breast duct is present (top left). HE 400x (inset). d) SMA 100x. The tumour shows negative staining for myoepithelial cells with SMA. Strong positive staining is seen in the myoepithelial cells of the normal breast duct (top left). Staining is also seen in stromal blood vessels. SMA 400x (inset) – Negative.
The patient was discussed in the post-operative MDM and a review of the published cases was performed. A recommendation was made for an ENT referral to exclude breast metastasis from salivary primary and a delayed sentinel lymph node biopsy to exclude lymphatic spread. A flexible nasal endoscope examination showed no primary index tumour in the upper aerodigestive tract and an US scan of the neck was unremarkable. No metastatic deposits were identified in the sentinel nodes removed.
II Literature Review
The PubMed search produced several papers, but we identified only 26 authors that had published cases that were absolutely related to our case, most focusing on the pathological aspect [1-9, 11-28]. This reflects the fact that MEC of the breast is a rare lesion and rather underreported.
Deriving from our analysis of the published cases (Tables 1 & 2), the mean age of all reported cases was 57 (SD 15.2), with a range from 27 to 86. Not all authors reported the medical history of the patients, but among the cases for which we have information, the majority described a palpable lump in their breasts for a few months, up to 37 years [4-9, 14, 18, 24, 27]. In 3 cases, the complaint was nipple discharge and in only two cases, including our case, the lesion was identified in screening mammograms [1]. The majority of the reported cases were on the left side (55.6%) and less on the right side (44.4%), but that was incidental with no clinical importance or explanation (p=0.56).
The size of the lesions ranged between 5-82 mm, with a median size of 20 mm (Q1-3: 13-35 mm). In our case, the cystic lesion that initially was identified, measured 12x8 mm, and although it was aspirated to dryness, it recurred and presented as a complex cyst 3 years later measuring 24x12 mm.
As most of the publications do not provide radiological imaging, it is difficult to interpret the description that was reported. From the patient of which we have information about their mammograms, in one case, there was a mass with microcalcifications, in 5 cases, there was a mass highly suspicious for malignancy and in 3 cases (including ours) a round lesion was seen without suspicious features [2-5, 7, 9, 13, 23, 27].
Table 1: Patient demographics and tumour characteristics among the published cases.
|
Age |
|
|
Mean (SD) |
57 (15.2) |
|
Median (Q1-3) |
57 (49-69) |
|
Range |
27-86 |
|
Side |
|
|
Left |
15 |
|
Right |
12 |
|
Size - mm |
|
|
Mean (SD) |
29.5mm (23.6) |
|
Median (Q1-3) |
20mm (13-45) |
|
Range |
5-82mm |
|
Grade |
|
|
High |
15 |
|
Intermediate |
3 |
|
Low |
20* |
|
NA |
3 |
*one case was low-grade that recurred as high-grade [22] SD: standard deviation, Q: quartile, mm: millimeters, NA: not applicable.
On US examination, 3 cases were reported as a hypoechoic mass with irregular margins, 1 case as a heterogeneous lesion with irregular margins, 2 cases as a nodule with well-defined margins, 2 have been described as a nodule with obscure boundary, one as an irregular solid-cystic mass, and 2 (including our case) as a well-defined complex cystic lesion [2, 3, 5-9].
Only 2 of the reported cases had MRI examination [2, 3]. Horri described that MRI identified a homogenously enhancing mass. In the case reported by Fujino, the MEC was predominantly hyperechoic in the US with a hypoechoic area inside the lesion that corresponded to an irregular, highly enhancing lesion in the MRI with a focal non-enhancing area within the lesion, that could be part of a cyst or necrosis. Surprisingly, in one case published by Palermo, the patient had surgery without previous imaging [27]. They did not report the reason for that, but maybe because of the patient’s age (80 years), the preoperative breast imaging assessment might not have been possible or accessible.
Table 2: Summary of previously reported cases of mucoepidermoid carcinoma of the breast.
|
No |
Authors |
Year Published |
Age |
Grade |
Size (mm) |
Type of surgery |
Medical Approach |
LN procedure |
No of LN |
LN Status |
Follow up (m) |
Status |
Distant Metastasis |
|
1 |
Patchefsky 1 [19] |
1979 |
66 |
Low |
13 |
Radical Mastectomy |
NS |
AC |
0/ (20) |
Negative |
94 |
Died -other causes |
NS |
|
2 |
Patchefsky 2 [19] |
1979 |
70 |
Low |
50 |
Quadrectomy |
NS |
NS |
NS |
NS |
10 |
Alive |
NS |
|
3 |
Kovi [15] |
1981 |
46 |
High |
NA |
Radical Mastectomy |
NS |
AC |
17/ (19) |
Positive |
NS |
NS |
NS |
|
4 |
Fisher 1 [12] |
1983 |
65 |
Low |
NA |
WLE |
NS |
NS |
NS |
NS |
60 |
Alive |
NS |
|
5 |
Fisher 2 [12] |
1983 |
71 |
Low |
NA |
Radical Mastectomy |
NS |
AC |
0 / (19) |
Negative |
48 |
Alive |
NS |
|
6 |
Fisher 3 [12] |
1983 |
57 |
Low |
NA |
Radical Mastectomy |
NS |
AD |
0/ (11) |
Negative |
120 |
Alive |
NS |
|
7 |
Fisher 4 [12] |
1983 |
49 |
Low |
NA |
Radical Mastectomy |
NS |
AD |
0/ (13) |
Negative |
108 |
Alive |
NS |
|
8 |
Fisher 5 [12] |
1983 |
60 |
Low |
NA |
Radical Mastectomy |
NS |
NS |
NS |
NS |
48 |
Died -other causes |
NS |
|
9 |
Ratanarapee [21] |
1983 |
27 |
High |
NA |
Radical Mastectomy |
NS |
AD |
6/ (15) |
Positive |
14 |
Died -other causes |
NS |
|
10 |
Luchtrath [17] |
1984 |
81 |
Low |
NS |
Mastectomy |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
|
11 |
Hanna 1 [13] |
1985 |
51 |
NA |
20 |
Modified Radical Mastectomy |
none |
NS |
0/ (ns) |
Negative |
8 |
Alive |
NS |
|
12 |
Hanna 2 [13] |
1985 |
31 |
NA |
NA |
Modified Radical Mastectomy |
Chemotherapy |
AC |
2/ (18) |
Positive |
14 |
Alive |
NS |
|
13 |
Hastrup [14] |
1985 |
59 |
High |
10 |
Modified Radical Mastectomy |
* |
SLNB |
0/ (4) |
Negative |
25 |
Died |
Recurrence Same Breast and New primary Controlateral Breast |
|
14 |
Leong [16] |
1985 |
57 |
High |
|
Radical Mastectomy |
NS |
AC |
0/ (20) |
Negative |
6 |
Died |
lung and spine |
|
15 |
Luchtrath [18] |
1989 |
60 |
High |
50 |
Radical Mastectomy |
Chemotherapy |
AC |
12/ (18) |
Positive |
30 |
Died |
Bone |
|
16 |
Pettinato [20] |
1989 |
72 |
High |
NA |
Mastectomy |
NS |
AC |
16/ (19) |
Positive |
few |
Died |
NS |
|
17 |
Berry [7] |
1996 |
51 |
High |
NA |
Mastectomy and primary reconstruction |
NS |
AC |
0/ (NS) |
Negative |
NS |
Alive |
NS |
|
18 |
Chang [11] |
1998 |
54 |
High |
NA |
Modified Radical Mastectomy |
Chemotherapy |
AD |
0/ (9) |
Negative |
48 |
Alive |
No |
|
19 |
Markopoulos [4] |
1998 |
40 |
High |
20 |
WLE |
Chemotherapy, Radiotherapy, Tamoxifen |
AD |
0/ (Level 1) |
Negative |
60 |
Alive |
No |
|
20 |
Tjalma [22] |
2002 |
49 |
low that recurrent as high |
35 |
Radical Mastectomy |
NS |
AC |
1/ (17) |
Positive |
156 |
Alive |
NS |
|
21 |
Di Tommaso 1 [26] |
2003 |
80 |
Low |
5 |
WLE |
NS |
NP |
NP |
NP |
5 |
Alive |
NS |
|
22 |
Di Tommaso 2 [26] |
2003 |
29 |
Low |
8 |
WLE |
NS |
NP |
NP |
NP |
90 |
Alive |
NS |
|
23 |
Di Tommaso 3 [26] |
2003 |
54 |
Low |
15 |
WLE |
NS |
AD |
0/ (NS) |
Negative |
13 |
Alive |
NS |
|
24 |
Di Tommaso 4 [26] |
2003 |
55 |
Intermediate |
6 |
WLE |
NS |
AD |
0/ (NS) |
Negative |
3 |
Alive |
NS |
|
25 |
Di Tommaso 5 [26] |
2003 |
36 |
High |
11 |
Quadrectomy |
NS |
AD |
0/ (NS) |
Negative |
18 |
Alive |
NS |
|
26 |
Aysen [23] |
2004 |
79 |
High |
80 |
Radical Mastectomy |
NS |
AD |
4/ (14) |
Positive |
NS |
NS |
NS |
|
27 |
Gomez Aracil [9] |
2006 |
69 |
High |
75 |
Radical Mastectomy |
Chemotherapy neo-adjuvant |
AC |
24/ (28) |
Positive |
NS |
NS |
NS |
|
28 |
Horii [3] |
2006 |
54 |
Low |
25 |
Mastectomy |
Aromatase Inhibitors |
AD |
0/ (NS) |
Negative |
36 |
Alive |
No |
|
29 |
Hornychova [1] |
2006 |
63 |
High |
18 |
Partial mastectomy |
Chemotherapy Radiotherapy |
AD |
0/ (17) |
Negative |
18 |
Alive |
No |
|
30 |
Hornychova [1] |
2006 |
30 |
Low |
82 |
Mastectomy |
Chemotherapy Radiotherapy |
AD |
0/ (NS) |
Negative |
60 |
Alive |
NS |
|
31 |
Camelo Piragua [25] |
2008 |
49 |
Intermediate |
15 |
Modified Radical Mastectomy |
Chemotherapy |
SLNB |
1/ (3+9) |
Positive |
8 |
Alive |
No |
|
32 |
Basbug [24] |
2011 |
69 |
High |
10 |
Mastectomy |
Chemotherapy Radiotherapy |
AD |
0/ (12) |
Negative |
12 |
Alive |
No |
|
33 |
Palermo [27] |
2013 |
80 |
High |
40 |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
|
34 |
Turk [5] |
2013 |
40 |
NS |
55 |
Modified Radical Mastectomy |
Chemotherapy |
AC |
1/ (24) |
Positive |
5 |
Alive |
No |
|
35 |
Fujino [2] |
2016 |
71 |
Intermediate |
17 |
Mastectomy |
NS |
SLNB |
NS |
NS |
NS |
NS |
NS |
|
36 |
Sherwell-Cabello [6, 28] |
2016 and 2017** |
86 |
Low |
60 |
Modified Radical Mastectomy |
No |
NS |
NS |
NS |
3 |
Alive |
No |
|
37 |
Cheng 1 [8] |
2017 |
39 |
Low |
15 |
Modified Radical Mastectomy |
NS |
AC |
3/ (18) |
Positive |
NS |
Alive |
NS |
|
38 |
Cheng 2 [8] |
2017 |
49 |
Low |
15 |
Modified Radical Mastectomy |
NS |
AC |
0/ (17) |
Negative |
NS |
Alive |
NS |
|
39 |
Cheng 3 [8] |
2017 |
66 |
Low |
13 |
Mastectomy |
NS |
SLNB |
0/ (6) |
Negative |
NS |
Alive |
NS |
|
40 |
Cheng 4 [8] |
2017 |
61 |
Low |
30 |
Mastectomy |
NS |
SLNB |
0/ (3) |
Negative |
NS |
Alive |
NS |
|
41 |
Our |
2018 |
63 |
Low |
21 |
WLE |
NS |
SLNB |
0/ (5) |
Negative |
36 |
Alive |
NS |
*None at presentation. Chemotherapy, Radiotherapy and Endocrine Treatment was given when the malignancy recurred with metastasis **The same case was reported in 2 different papers by the same author. NS: not stated AC: axillary node clearance, AD: axillary dissection SLNB: sentinel lymph node biopsy, NA: not applicable, No: number, NP: not performed, WLE: wide local excision
Preoperative biopsy under US or stereo-guidance is standard practice. Nowadays, the benefits of core biopsy over fine-needle aspiration cytology (FNAC) are well-known. However, as most of the cases that have been published in the literature are old cases, most did not undergo preoperative biopsy, or were lacking information. Only 12 cases reported a type of procedure, including FNAC (n=7) and a core biopsy (n=5). In all 5 cases that had a core biopsy, malignancy was reported, but it was not specified that it was a case of mucoepidermoid malignancy [1, 2, 5, 6, 27]. From those that had FNAC, in 4 cases, cancerous cells were identified, 1 case was reported as C3 and 1 case was reported as C2, although macroscopically it was reported as dark hemorrhagic fluid [3, 7, 9, 20, 24, 27]. Our case is an interesting one as the initial FNA obtained a blood-stained fluid that in the cytology reported as C2 and no further biopsies were taken. However, 3-years later, a core biopsy on a recurrent lesion performed and a mucoepidermoid carcinoma was suspected and graded as B4.
Table 3: Review of the surgical treatment in the published cases.
|
Type of Surgery |
No of Patients |
|
Radical Mastectomy |
12 |
|
Modified Radical Mastectomy |
8 |
|
Mastectomy |
8 |
|
Mastectomy and primary reconstruction |
1 |
|
Partial mastectomy |
1 |
|
Quadrectomy |
2 |
|
Wide Local Excision |
7 |
|
NS |
2 |
|
Grand Total |
41 |
No: number. NS: not state
Breast conserving surgery has become well-established in the treatment of early breast cancer, but this has only taken place systemically in the last 15 years. In the current case series review, the majority of the patients (29/41) (Tables 2 & 3) underwent mastectomy, independent of the grading or the size of the malignancy and only 10/41 patients had a smaller procedure, such as wide local excision or quadrantectomy. We would expect in the newer published series, such as Cheng’s, to see cases of conservative surgeries, but surprisingly, all 4 cases that are reported had mastectomies, although the sizes of the lesions were 13, 15, 15 and 30 mm [8]. One explanation might be that as the collection of the cases goes back to 2004, the surgical practice at this stage was different.
Similarly, most of the cases had a type of axillary lymph node procedure (32/41) independent of the histological grade or the size of the malignancy or the preoperative radiological assessment. 2/41 cases had no axillary dissection, while for 7 cases, we have no information. In the high-grade cases (n=15) 6 were positive for metastasis, in the intermediate-grade cases (n=3) one was positive and in the low-grade cases (n=19 plus one case that was initially presented as low grade and recurred as high grade) 2 were positive for metastasis (Table 4).
The tumors’ grade is based on the Elston-Ellis grading system for breast carcinoma and, variously, on the AFIP Auclair and Brandwein grading systems for MEC of salivary glands [29-31]. The pathologic grading systems differ in their assessment criteria and nomenclature: Elston-Ellis resulting in grade 1, 2 and 3 carcinomas and AFIP and Brandwein grouping into low, intermediate and high-grade. Neither system has been validated in mucoepidermoid carcinomas of the breast due to their rarity and the inaccurate interchangeable use of both in the published literature. The criteria for grading in the salivary glands have been applied to those of the breast and appears to correlate well with lymph node metastases and survival [7]. In the published cases, it is unclear what grading system was used. 15 cases were reported as High-grade, 3 cases as Intermediated grade, 19 cases (including our case) as low-grade, while for 3 cases, we have no information. Interestingly, one of the cases of low-grade mucoepidermoid carcinoma, published by Tjalma, the carcinoma recurred as high-grade, 32 months after the initial treatment and furthermore, the patient developed a poorly differentiated adenocarcinoma in the contralateral breast, 12 years postoperatively [22]. Our case was a low-grade mucoepidermoid carcinoma in a background of DCIS.
About the receptor’s status (Table 5), we have information only for 18/41 cases. Although those tumors are considered to be estrogen receptor (ER) negative, in the published cases, 55.5% (10/18) were ER-negative and 44.4% (8/18) ER-positive. HER-2 was negative in all 10 cases that we have information on the HER-2 status.
Additional useful diagnostic immunohistochemical staining was reported as follow: For p63, information was available for 13 cases: 8 of which were positive, 2 were weak or incompletely expressed and 3 were negative. For CK5/6, we have reports for 9 cases, of which 2 were negative and 9 positive. For CK7 we have data for 13 cases, of which 10 were positive, 2 were negative and 1 was reported as weak. Finally, for Ki-67, we have information from 5 published cases, with proliferation fractions of 40, 25, 22 % and 5% and one was reported as “positive" [1, 2, 6, 9]. Our case had a proliferation fraction of 5%.
Table 4: Review of the axillary procedures that were performed based on the histopathology grading of the tumor, in the published cases.
|
|
Tumor Grading |
|
||||
|
Axillary Procedure Axillary Status |
High |
Intermediate |
Low |
Low - High Recurrence |
NA/NS |
Total |
|
Axillary Clearance |
6 |
4 |
1 |
2 |
13 |
|
|
Positive |
4 |
1 |
1 |
1 |
8 |
|
|
Negative |
2 |
3 |
5 |
|||
|
Axillary Dissection |
7 |
1 |
5 |
13 |
||
|
Positive |
2 |
2 |
||||
|
Negative |
5 |
1 |
5 |
11 |
||
|
SLNB |
1 |
2 |
3 |
6 |
||
|
Positive |
1 |
1 |
||||
|
Negative |
1 |
3 |
4 |
|||
|
NS |
1 |
1 |
||||
|
No Axillary Dissection |
2 |
2 |
||||
|
Not stated |
1 |
5 |
1 |
7 |
||
|
Grand Total |
15 |
3 |
19 |
1 |
3 |
41 |
NA: not applicable, NS: not stated, SLNB: sentinel lymph node biopsy.
Table 5: Review of the grading and immunohistochemical profiles of the different published cases.
|
|
Grade |
ER |
HER2+ |
P 63 |
CK 5/6 |
CK 7 |
Ki-67 |
|
Hanna [13] |
NA |
+ |
NS |
NS |
NS |
NS |
NS |
|
Hanna [13] |
NA |
+ |
NS |
NS |
NS |
NS |
NS |
|
Hastrup[14] |
High |
- |
NS |
NS |
NS |
NS |
NS |
|
Markopoulos[4] |
High |
- |
NS |
NS |
NS |
NS |
NS |
|
Gomez Aracil [9] |
High |
+ |
NS |
NS |
NS |
+ |
positive |
|
Horii[3] |
Low |
+ |
1+ |
NS |
NS |
NS |
NS |
|
Hornychova[1] |
High |
- |
- |
20% |
+ |
weak |
25% |
|
Hornychova[1] |
Low |
- |
- |
15% |
+ |
+ |
40% |
|
Basbug [24] |
High |
- |
- |
NS |
NS |
NS |
NS |
|
Palermo[27] |
High |
- |
NS |
+ |
|
+ |
|
|
Fujino[2] |
Intermediate |
- |
- |
+ |
+ |
+ |
22% |
|
Turk[5] |
NS |
- |
NS |
NS |
NS |
+ |
NS |
|
Sherwell-Cabello[6, 28] |
Low |
- |
- |
+ |
+ |
+ |
5% |
|
Cheng[8] |
Low |
+ |
- |
- |
- |
- |
NS |
|
Cheng[8] |
Low |
- |
- |
- |
+ |
+ |
NS |
|
Cheng[8] |
Low |
10% |
- |
- |
- |
- |
NS |
|
Cheng[8] |
Low |
60% |
- |
+ |
+ |
+ |
NS |
|
Our |
Low |
+ |
NS |
+ |
+ |
+ |
5% |
ER: estrogen, HER2: human epidermal growth factor receptor 2, CK: cytokeratin, NA: not applicable, NS: not stated.
Concerning medical treatment, from the 41 cases, 30 patients did not receive chemotherapy or there was no statement about this. 9 patients had chemotherapy and the majority of those were cases of high-grade mucoepidermoid carcinoma, one patient received neoadjuvant chemotherapy and one received chemotherapy at the onset of recurrence [9, 14]. The majority of the low-grade cases did not have chemotherapy. 5 patients reported that they received radiotherapy and 4 of those were cases of high-grade MEC [1, 4, 18, 24]. As our patient was a case of low-grade MEC, the MDM decision was to omit chemotherapy, endocrine treatment and radiotherapy.
The median follow-up time after the diagnosis in the published cases was 20 months (Q1-3: 13-45). From the 41 cases, we had reports that 5 patients died because of their MEC [14, 16, 18, 20, 21]. All of them had High-grade MEC (5 patients out of 15 that had high-grade MEC) and 3 of those had positive lymph nodes (16, 12 and 6 positive lymph nodes in the axillary clearance) at the time of the primary treatment.
Discussion
Mucoepidermoid carcinoma is a tumor that predominantly arises from the major salivary glands and less commonly from minor salivary glands, of the oral and nasal cavity. There are reports of these tumors arising in the esophagus and bronchial tree, thyroid gland, thymus, ear and mandible, pancreas, lacrimal gland and skin adnexa [1].
Breast is a rare location for MEC and in an extended review of the literature, going back to 1970’s, we found that up to date, only 40 cases have been reported (41 with ours)(Table 1). Cheng reports the incidence of MEC in the breast to be 0.03% but Fisher believes that it is 0.2-0.3% or even higher because these lesions may masquerade under other diagnoses, such as atypical squamous metaplasia [8, 12]. Both breast and major salivary glands arise from the embryonal ectoderm and thus share the same morphological and immunohistochemical features [8, 13, 26].
Primary MEC of the breast is extremely rare. Our case is one of the 41 reported cases of MEC cases and 1 of the 20 low-grade cases. Usually, the patients are over 50 (mean age 57) and the lesion is relatively big (median size 20mm) and symptomatic. In a thorough review of the published cases, we concluded that the patients seek medical advice when a palpable mass is present. Nipple discharge is unusual, while only 2 cases have been reported as asymptomatic and were diagnosed during the breast screening program.
Imaging features of rare breast lesions can be unfamiliar to the breast radiologist due to their scarcity and the diagnostic and therapeutic approach is not well-documented in the literature. Imaging characteristics may vary. It can appear as a mass with irregular margins, as a nodule with well-circumscribed margins or even mimic a simple or a complex cyst in the US examination. The presence of calcifications is rare and only one case has been reported [2]. Our case was a cyst like lesion, that can easily be mistaken for a benign lesion and this is why the final diagnosis was delayed 3 years. MRI characteristics are not typical. It might show a homogenously enhancing mass or an irregular, highly enhancing lesion with a focal non-enhancing area within the lesion, that might be a part of a cyst or necrosis [2, 3].
Preoperative biopsy under US or stereo-guidance is standard practice. Nowadays, the benefits of the core biopsy over fine-needle aspiration cytology (FNAC) are well-known and it should be performed to all the indeterminate or suspicious lesions seen in the mammograms and US examinations. FNAC can lead to false negative or indeterminate findings, causing delays in the diagnosis, as in our case.
Given the low incidence of MEC, there is a lack of consensus for treatment. However, based on the size, the grading of the tumor and the nodal status on the preoperative assessment, breast conservative surgery is preferred with or without axillary sentinel node biopsy, similarly to the other type of malignancies in the breast. Since the management and treatment of the axilla is moving towards conservatism, in low-grading MEC with negative preoperative assessment of the axilla, a SLNB could be avoided. In the literature we identified only 2 /41 cases that were diagnosed with metastasis.
MEC are considered to be ER and HER-2 negative. In low-grade carcinomas, radiotherapy, chemotherapy and endocrine treatment is not indicated, but it may be considered in cases with High-grade morphology, measuring over 3 cm in size or those which are positive for metastasis [28]. The prognosis of MEC is relatively good and is better for the low-grade compared to the high-grade MEC, with low risk of metastasis or recurrence.
Due to its rarity, the diagnostic and therapeutic approach of MEC in the breast is not well-documented, causing challenges in daily practice, even at experienced Breast Centers. A review of the published cases is essential for the multidisciplinary team to plan treatment strategies.
Conflicts of Interest
None.
Funding
None.
Abbreviations
MEC: Mucoepidermoid carcinoma
MDM: multidisciplinary meeting
FNAc: fine needle aspiration-cytology
ACR: American College of Radiology
BIRADS: Breast Imaging Reporting and Data System
MLO: mediolateral oblique
CC: craniocaudal
Article Info
Article Type
Case Report and Review of the LiteraturePublication history
Received: Tue 07, Apr 2020Accepted: Wed 22, Apr 2020
Published: Wed 29, Apr 2020
Copyright
© 2023 Linda Metaxa. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.COR.2020.04.08
Author Info
Georgios Exarchos Linda Metaxa Louise J. Jones Philip Elliott Philip Dilks Shefali Dani Taha Sewedy Tamara D. Suaris
Corresponding Author
Linda MetaxaBreast Imaging Department, St Bartholomew's Hospital, London, UK
Figures & Tables
Table 1: Patient demographics and tumour characteristics among the published cases.
|
Age |
|
|
Mean (SD) |
57 (15.2) |
|
Median (Q1-3) |
57 (49-69) |
|
Range |
27-86 |
|
Side |
|
|
Left |
15 |
|
Right |
12 |
|
Size - mm |
|
|
Mean (SD) |
29.5mm (23.6) |
|
Median (Q1-3) |
20mm (13-45) |
|
Range |
5-82mm |
|
Grade |
|
|
High |
15 |
|
Intermediate |
3 |
|
Low |
20* |
|
NA |
3 |
*one case was low-grade that recurred as high-grade [22] SD: standard deviation, Q: quartile, mm: millimeters, NA: not applicable.
Table 2: Summary of previously reported cases of mucoepidermoid carcinoma of the breast.
|
No |
Authors |
Year Published |
Age |
Grade |
Size (mm) |
Type of surgery |
Medical Approach |
LN procedure |
No of LN |
LN Status |
Follow up (m) |
Status |
Distant Metastasis |
|
1 |
Patchefsky 1 [19] |
1979 |
66 |
Low |
13 |
Radical Mastectomy |
NS |
AC |
0/ (20) |
Negative |
94 |
Died -other causes |
NS |
|
2 |
Patchefsky 2 [19] |
1979 |
70 |
Low |
50 |
Quadrectomy |
NS |
NS |
NS |
NS |
10 |
Alive |
NS |
|
3 |
Kovi [15] |
1981 |
46 |
High |
NA |
Radical Mastectomy |
NS |
AC |
17/ (19) |
Positive |
NS |
NS |
NS |
|
4 |
Fisher 1 [12] |
1983 |
65 |
Low |
NA |
WLE |
NS |
NS |
NS |
NS |
60 |
Alive |
NS |
|
5 |
Fisher 2 [12] |
1983 |
71 |
Low |
NA |
Radical Mastectomy |
NS |
AC |
0 / (19) |
Negative |
48 |
Alive |
NS |
|
6 |
Fisher 3 [12] |
1983 |
57 |
Low |
NA |
Radical Mastectomy |
NS |
AD |
0/ (11) |
Negative |
120 |
Alive |
NS |
|
7 |
Fisher 4 [12] |
1983 |
49 |
Low |
NA |
Radical Mastectomy |
NS |
AD |
0/ (13) |
Negative |
108 |
Alive |
NS |
|
8 |
Fisher 5 [12] |
1983 |
60 |
Low |
NA |
Radical Mastectomy |
NS |
NS |
NS |
NS |
48 |
Died -other causes |
NS |
|
9 |
Ratanarapee [21] |
1983 |
27 |
High |
NA |
Radical Mastectomy |
NS |
AD |
6/ (15) |
Positive |
14 |
Died -other causes |
NS |
|
10 |
Luchtrath [17] |
1984 |
81 |
Low |
NS |
Mastectomy |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
|
11 |
Hanna 1 [13] |
1985 |
51 |
NA |
20 |
Modified Radical Mastectomy |
none |
NS |
0/ (ns) |
Negative |
8 |
Alive |
NS |
|
12 |
Hanna 2 [13] |
1985 |
31 |
NA |
NA |
Modified Radical Mastectomy |
Chemotherapy |
AC |
2/ (18) |
Positive |
14 |
Alive |
NS |
|
13 |
Hastrup [14] |
1985 |
59 |
High |
10 |
Modified Radical Mastectomy |
* |
SLNB |
0/ (4) |
Negative |
25 |
Died |
Recurrence Same Breast and New primary Controlateral Breast |
|
14 |
Leong [16] |
1985 |
57 |
High |
|
Radical Mastectomy |
NS |
AC |
0/ (20) |
Negative |
6 |
Died |
lung and spine |
|
15 |
Luchtrath [18] |
1989 |
60 |
High |
50 |
Radical Mastectomy |
Chemotherapy |
AC |
12/ (18) |
Positive |
30 |
Died |
Bone |
|
16 |
Pettinato [20] |
1989 |
72 |
High |
NA |
Mastectomy |
NS |
AC |
16/ (19) |
Positive |
few |
Died |
NS |
|
17 |
Berry [7] |
1996 |
51 |
High |
NA |
Mastectomy and primary reconstruction |
NS |
AC |
0/ (NS) |
Negative |
NS |
Alive |
NS |
|
18 |
Chang [11] |
1998 |
54 |
High |
NA |
Modified Radical Mastectomy |
Chemotherapy |
AD |
0/ (9) |
Negative |
48 |
Alive |
No |
|
19 |
Markopoulos [4] |
1998 |
40 |
High |
20 |
WLE |
Chemotherapy, Radiotherapy, Tamoxifen |
AD |
0/ (Level 1) |
Negative |
60 |
Alive |
No |
|
20 |
Tjalma [22] |
2002 |
49 |
low that recurrent as high |
35 |
Radical Mastectomy |
NS |
AC |
1/ (17) |
Positive |
156 |
Alive |
NS |
|
21 |
Di Tommaso 1 [26] |
2003 |
80 |
Low |
5 |
WLE |
NS |
NP |
NP |
NP |
5 |
Alive |
NS |
|
22 |
Di Tommaso 2 [26] |
2003 |
29 |
Low |
8 |
WLE |
NS |
NP |
NP |
NP |
90 |
Alive |
NS |
|
23 |
Di Tommaso 3 [26] |
2003 |
54 |
Low |
15 |
WLE |
NS |
AD |
0/ (NS) |
Negative |
13 |
Alive |
NS |
|
24 |
Di Tommaso 4 [26] |
2003 |
55 |
Intermediate |
6 |
WLE |
NS |
AD |
0/ (NS) |
Negative |
3 |
Alive |
NS |
|
25 |
Di Tommaso 5 [26] |
2003 |
36 |
High |
11 |
Quadrectomy |
NS |
AD |
0/ (NS) |
Negative |
18 |
Alive |
NS |
|
26 |
Aysen [23] |
2004 |
79 |
High |
80 |
Radical Mastectomy |
NS |
AD |
4/ (14) |
Positive |
NS |
NS |
NS |
|
27 |
Gomez Aracil [9] |
2006 |
69 |
High |
75 |
Radical Mastectomy |
Chemotherapy neo-adjuvant |
AC |
24/ (28) |
Positive |
NS |
NS |
NS |
|
28 |
Horii [3] |
2006 |
54 |
Low |
25 |
Mastectomy |
Aromatase Inhibitors |
AD |
0/ (NS) |
Negative |
36 |
Alive |
No |
|
29 |
Hornychova [1] |
2006 |
63 |
High |
18 |
Partial mastectomy |
Chemotherapy Radiotherapy |
AD |
0/ (17) |
Negative |
18 |
Alive |
No |
|
30 |
Hornychova [1] |
2006 |
30 |
Low |
82 |
Mastectomy |
Chemotherapy Radiotherapy |
AD |
0/ (NS) |
Negative |
60 |
Alive |
NS |
|
31 |
Camelo Piragua [25] |
2008 |
49 |
Intermediate |
15 |
Modified Radical Mastectomy |
Chemotherapy |
SLNB |
1/ (3+9) |
Positive |
8 |
Alive |
No |
|
32 |
Basbug [24] |
2011 |
69 |
High |
10 |
Mastectomy |
Chemotherapy Radiotherapy |
AD |
0/ (12) |
Negative |
12 |
Alive |
No |
|
33 |
Palermo [27] |
2013 |
80 |
High |
40 |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
|
34 |
Turk [5] |
2013 |
40 |
NS |
55 |
Modified Radical Mastectomy |
Chemotherapy |
AC |
1/ (24) |
Positive |
5 |
Alive |
No |
|
35 |
Fujino [2] |
2016 |
71 |
Intermediate |
17 |
Mastectomy |
NS |
SLNB |
NS |
NS |
NS |
NS |
NS |
|
36 |
Sherwell-Cabello [6, 28] |
2016 and 2017** |
86 |
Low |
60 |
Modified Radical Mastectomy |
No |
NS |
NS |
NS |
3 |
Alive |
No |
|
37 |
Cheng 1 [8] |
2017 |
39 |
Low |
15 |
Modified Radical Mastectomy |
NS |
AC |
3/ (18) |
Positive |
NS |
Alive |
NS |
|
38 |
Cheng 2 [8] |
2017 |
49 |
Low |
15 |
Modified Radical Mastectomy |
NS |
AC |
0/ (17) |
Negative |
NS |
Alive |
NS |
|
39 |
Cheng 3 [8] |
2017 |
66 |
Low |
13 |
Mastectomy |
NS |
SLNB |
0/ (6) |
Negative |
NS |
Alive |
NS |
|
40 |
Cheng 4 [8] |
2017 |
61 |
Low |
30 |
Mastectomy |
NS |
SLNB |
0/ (3) |
Negative |
NS |
Alive |
NS |
|
41 |
Our |
2018 |
63 |
Low |
21 |
WLE |
NS |
SLNB |
0/ (5) |
Negative |
36 |
Alive |
NS |
*None at presentation. Chemotherapy, Radiotherapy and Endocrine Treatment was given when the malignancy recurred with metastasis **The same case was reported in 2 different papers by the same author. NS: not stated AC: axillary node clearance, AD: axillary dissection SLNB: sentinel lymph node biopsy, NA: not applicable, No: number, NP: not performed, WLE: wide local excision
Table 3: Review of the surgical treatment in the published cases.
|
Type of Surgery |
No of Patients |
|
Radical Mastectomy |
12 |
|
Modified Radical Mastectomy |
8 |
|
Mastectomy |
8 |
|
Mastectomy and primary reconstruction |
1 |
|
Partial mastectomy |
1 |
|
Quadrectomy |
2 |
|
Wide Local Excision |
7 |
|
NS |
2 |
|
Grand Total |
41 |
No: number. NS: not state
Table 4: Review of the axillary procedures that were performed based on the histopathology grading of the tumor, in the published cases.
|
|
Tumor Grading |
|
||||
|
Axillary Procedure Axillary Status |
High |
Intermediate |
Low |
Low - High Recurrence |
NA/NS |
Total |
|
Axillary Clearance |
6 |
4 |
1 |
2 |
13 |
|
|
Positive |
4 |
1 |
1 |
1 |
8 |
|
|
Negative |
2 |
3 |
5 |
|||
|
Axillary Dissection |
7 |
1 |
5 |
13 |
||
|
Positive |
2 |
2 |
||||
|
Negative |
5 |
1 |
5 |
11 |
||
|
SLNB |
1 |
2 |
3 |
6 |
||
|
Positive |
1 |
1 |
||||
|
Negative |
1 |
3 |
4 |
|||
|
NS |
1 |
1 |
||||
|
No Axillary Dissection |
2 |
2 |
||||
|
Not stated |
1 |
5 |
1 |
7 |
||
|
Grand Total |
15 |
3 |
19 |
1 |
3 |
41 |
NA: not applicable, NS: not stated, SLNB: sentinel lymph node biopsy.
Table 5: Review of the grading and immunohistochemical profiles of the different published cases.
|
|
Grade |
ER |
HER2+ |
P 63 |
CK 5/6 |
CK 7 |
Ki-67 |
|
Hanna [13] |
NA |
+ |
NS |
NS |
NS |
NS |
NS |
|
Hanna [13] |
NA |
+ |
NS |
NS |
NS |
NS |
NS |
|
Hastrup[14] |
High |
- |
NS |
NS |
NS |
NS |
NS |
|
Markopoulos[4] |
High |
- |
NS |
NS |
NS |
NS |
NS |
|
Gomez Aracil [9] |
High |
+ |
NS |
NS |
NS |
+ |
positive |
|
Horii[3] |
Low |
+ |
1+ |
NS |
NS |
NS |
NS |
|
Hornychova[1] |
High |
- |
- |
20% |
+ |
weak |
25% |
|
Hornychova[1] |
Low |
- |
- |
15% |
+ |
+ |
40% |
|
Basbug [24] |
High |
- |
- |
NS |
NS |
NS |
NS |
|
Palermo[27] |
High |
- |
NS |
+ |
|
+ |
|
|
Fujino[2] |
Intermediate |
- |
- |
+ |
+ |
+ |
22% |
|
Turk[5] |
NS |
- |
NS |
NS |
NS |
+ |
NS |
|
Sherwell-Cabello[6, 28] |
Low |
- |
- |
+ |
+ |
+ |
5% |
|
Cheng[8] |
Low |
+ |
- |
- |
- |
- |
NS |
|
Cheng[8] |
Low |
- |
- |
- |
+ |
+ |
NS |
|
Cheng[8] |
Low |
10% |
- |
- |
- |
- |
NS |
|
Cheng[8] |
Low |
60% |
- |
+ |
+ |
+ |
NS |
|
Our |
Low |
+ |
NS |
+ |
+ |
+ |
5% |
ER: estrogen, HER2: human epidermal growth factor receptor 2, CK: cytokeratin, NA: not applicable, NS: not stated.





References
- Hornychova H, Ryska A, Betlach J, Bohác R, Cízek T et al. (2007) Mucoepidermoid carcinoma of the breast. Neoplasma 54: 168-172. [Crossref]
- Fujino M, Mori D, Akashi M, Yamamoto H, Aibe H et al. (2016) Mucoepidermoid Carcinoma of the Breast Found during Treatment of Lymphoma. Case Rep Oncol 9: 806-814. [Crossref]
- Horii R, Akiyama F, Ikenaga M, Iwase T, Sakamoto G (2006) Muco-epidermoid carcinoma of the breast. Pathol Int 56: 549-553. [Crossref]
- Markopoulos C, Gogas H, Livaditou A, Floros D (1998) Mucoepidermoid carcinoma of the breast. Eur J Gynaecol Oncol 19: 291-293. [Crossref]
- Turk E, Karagulle E, Erinanc OH, Soy EA, Moray G (2013) Mucoepidermoid carcinoma of the breast. Breast J 19: 206-208. [Crossref]
- Sherwell Cabello S, Maffuz Aziz A, Rios Luna NP, Pozo Romero M, Lopez Jimenez PV et al. (2017) Primary mucoepidermoid carcinoma of the breast. Breast J 23: 753-755. [Crossref]
- Berry MG, Caldwell C, Carpenter R (1998) Mucoepidermoid carcinoma of the breast: a case report and review of the literature. Eur J Surg Oncol 24: 78-80. [Crossref]
- Cheng M, Geng C, Tang T, Song Z (2017) Mucoepidermoid carcinoma of the breast: Four case reports and review of the literature. Medicine (Baltimore) 96: e9385. [Crossref]
- Gomez Aracil V, Mayayo Artal E, Azua Romeo J, Mayayo Alvira R, Azua Blanco J et al. (2006) Fine needle aspiration cytology of high grade mucoepidermoid carcinoma of the breast: a case report. Acta Cytol 50: 344-348. [Crossref]
- Taylor K, Britton P, O'Keeffe S, Wallis MG (2011) Quantification of the UK 5-point breast imaging classification and mapping to BI-RADS to facilitate comparison with international literature. Br J Radiol 84: 1005-1010. [Crossref]
- Chang LC, Lee N, Lee CT, Huang JS (1998) High-grade mucoepidermoid carcinoma of the breast: case report. Changgeng Yi Xue Za Zhi 21: 352-357. [Crossref]
- Fisher ER, Palekar AS, Gregorio RM, Paulson JD (1983) Mucoepidermoid and squamous cell carcinomas of breast with reference to squamous metaplasia and giant cell tumors. Am J Surg Pathol 7: 15-27. [Crossref]
- Hanna W, Kahn HJ (1985) Ultrastructural and immunohistochemical characteristics of mucoepidermoid carcinoma of the breast. Hum Pathol 16: 941-946. [Crossref]
- Hastrup N, Sehested M (1985) High-grade mucoepidermoid carcinoma of the breast. Histopathology 9: 887-892.
- Kovi J, Duong HD, Leffall LS Jr. (1981) High-grade mucoepidermoid carcinoma of the breast. Arch Pathol Lab Med 105: 612-614. [Crossref]
- Leong AS, Williams JA (1985) Mucoepidermoid carcinoma of the breast: high grade variant. Pathology 17: 516-521. [Crossref]
- Luchtrath H (1984) Mucoepidermoid cancer of the breast]. Pathologe 5: 282-284. [Crossref]
- Luchtrath H, Moll R (1989) Mucoepidermoid mammary carcinoma. Immunohistochemical and biochemical analyses of intermediate filaments. Virchows Arch A Pathol Anat Histopathol 416: 105-113. [Crossref]
- Patchefsky AS, Frauenhoffer CM, Krall RA, Cooper HS (1979) Low-grade mucoepidermoid carcinoma of the breast. Arch Pathol Lab Med 103: 196-198. [Crossref]
- Pettinato G, Insabato L, De Chiara A, Manco A, Petrella G (1989) High-grade mucoepidermoid carcinoma of the breast. Fine needle aspiration cytology and clinicopathologic study of a case. Acta Cytol 33: 195-200. [Crossref]
- Ratanarapee S, Prinyar Nussorn N, Chantarakul N, Pacharee P (1983) High-grade mucoepidermoid carcinoma of the breast. A case report. J Med Assoc Thai 66: 642-648. [Crossref]
- Tjalma WA, Verslegers IO, De Loecker PA, Van Marck EA (2002) Low and high grade mucoepidermoid carcinomas of the breast. Eur J Gynaecol Oncol 23: 423-425. [Crossref]
- Aysen Terzi, Arzu Sağlam, Aysegul Uner (2004) A 79 year-old woman with a mass in the right breast. Turk J Cancer 34: 38-39.
- Basbug M, Akbulut S, Arikanoglu Z, Sogutcu N, Firat U et al. (2011) Mucoepidermoid carcinoma in a breast affected by burn scars: comprehensive literature review and case report. Breast Care (Basel) 6: 293-297. [Crossref]
- Camelo Piragua SI, Habib C, Kanumuri P, Lago CE, Mason HS et al. (2009) Mucoepidermoid carcinoma of the breast shares cytogenetic abnormality with mucoepidermoid carcinoma of the salivary gland: a case report with molecular analysis and review of the literature. Hum Pathol 40: 887-892. [Crossref]
- Di Tommaso L, Foschini MP, Ragazzini T, Magrini E, Fornelli A et al. (2004) Mucoepidermoid carcinoma of the breast. Virchows Arch 444: 13-19. [Crossref]
- Palermo MH, Pinto MB, Zanetti JS, Ribeiro Silva A (2013) Primary mucoepidermoid carcinoma of the breast: a case report with immunohistochemical analysis and comparison with salivary gland mucoepidermoid carcinomas. Pol J Pathol 64: 210-215. [Crossref]
- Sherwell Cabello S, Maffuz Aziz A, Rios Luna NP, Bautista Pina V, Rodriguez Cuevas S (2016) Salivary gland-like breast carcinomas: An infrequent disease. Pathol Res Pract 212: 1034-1038. [Crossref]
- Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19: 403-410. [Crossref]
- Auclair PL, Goode RK, Ellis GL (1992) Mucoepidermoid carcinoma of intraoral salivary glands. Evaluation and application of grading criteria in 143 cases. Cancer 69: 2021-2030. [Crossref]
- Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B et al. (2001) Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol 25: 835-845. [Crossref]
