Published online Aug 6, 2020. doi: 10.12998/wjcc.v8.i15.3218
Peer-review started: February 8, 2020
First decision: May 22, 2020
Revised: June 15, 2020
Accepted: July 15, 2020
Article in press: July 15, 2020
Published online: August 6, 2020
Refractory gastrointestinal bleeding (GIB) secondary to gastrointestinal vascular malformations (GIVM) such as gastrointestinal angiodysplasia (GIAD) and gastric antral vascular ectasia (GAVE) remains challenging to treat when endoscopic therapy fails. Recently thalidomide has been suggested as a treatment option for refractory GIB.
To determine the outcome of patients treated with thalidomide for refractory GIB due to GIVM.
IRB approved, single center, retrospective review of electronic medical records from January 2012 to November 2018. Patients age > 18 years old, who had > 3 episodes of GIB refractory to medical or endoscopic therapy, and who had been treated with thalidomide for at least 3 mo were included. The primary endpoint was recurrence of GIB 6 mo after initiation of thalidomide.
Fifteen patients were included in the study, all with significant cardiac, hepatic, or renal comorbidities. The cause of GIB was GIAD in 10 patients and GAVE in 5 patients. Two patients were lost to follow up. Of the 13 patients followed, 38.5% (n = 5) had no recurrent GIB or transfusion requirement after treatment with thalidomide. Furthermore, 84.6% (n = 11) of patients had a reduction in transfusion requirements and hospitalizations for GIB. Thalidomide was discontinued in 2 patients due to cost (n = 1) and medication interaction (n = 1). Reported adverse reactions included fatigue (n = 3), neuropathy (n = 2), dizziness (n = 1), and constipation (n = 1). Six patients died during follow up due to unknown cause (n = 4) and sepsis (n = 2).
Thalidomide appears to be an effective treatment for refractory GIB due to GIAD or GAVE in a Western population with significant comorbidities.
Core tip: Gastrointestinal vascular malformations (GIVM) consist of several types such as gastrointestinal angiodysplasias (GIAD) and gastric antral vascular ectasias (GAVE). Refractory gastrointestinal bleeding secondary to GIVM such as GIAD and GAVE remains challenging to treat when endoscopic therapy fails. Thalidomide appears to be an effective treatment for refractory gastrointestinal bleeding due to GIAD or GAVE in a Western population with significant comorbidities.
- Citation: Bayudan AM, Chen CH. Thalidomide for refractory gastrointestinal bleeding from vascular malformations in patients with significant comorbidities. World J Clin Cases 2020; 8(15): 3218-3229
- URL: https://www.wjgnet.com/2307-8960/full/v8/i15/3218.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i15.3218
Gastrointestinal vascular malformations (GIVM) consist of several types such as gastrointestinal angiodysplasias (GIAD) and gastric antral vascular ectasias (GAVE). The pathogenesis of vascular malformations remains unclear, but is thought to be due to an imbalance of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), and anti-angiogenic properties leading to neovascularization[1]. In vitro studies have also demonstrated hypoxic conditions leading to increased expression of hypoxia-inducible-factor-1-α (HIF-1α) and HIF-2α, which upregulate VEGF and participate in the formation of GIVM[2]. Patients with GIVM can present with overt gastrointestinal bleeding (GIB) or iron deficiency anemia (IDA) due to chronic occult blood loss, and can be associated with significant morbidity and mortality.
GIAD are abnormal, dilated, tortuous vessels within the mucosal and submucosal layers and are the most common GIVM seen throughout the gastrointestinal tract. GIAD can account for up to 40% of occult GIB episodes[3]. Several chronic conditions have been associated with GIAD such as aortic stenosis, which can cause an acquired von Willbrand disease leading to increased GIB, also known as Heyde’s Syndrome. GIAD has also been shown to increase risk of GIB in patients with left ventricular assist devices (LVAD) as well as chronic renal failure.
GAVE is generally found in patients with chronic illnesses, such as liver disease, connective tissue diseases, and chronic renal failure. Bleeding from GAVE accounts for 4% of upper GIB and is commonly found in women. Endoscopically, GAVE appears as either punctate lesions throughout the stomach or as red lesions radiating from the pylorus in stripes, known as “watermelon stomach”. Generally, it is found in the antrum with rare presentations in the proximal stomach. Histologically, GAVE is characterized by vascular ectasia of mucosal capillaries, focal thrombosis, spindle cell proliferation, and fibrinolysis.
Endoscopic therapy primarily with argon plasma coagulation (APC) is the mainstay of treatment for GIVM. However, GIVM refractory to endoscopic treatment is common and medications such as octreotide[7], tranexamic acid[8,9], hormonal therapy (estrogen-progesterone)[10], and thalidomide[8,11-23] have all been studied in the management of recurrent GIB refractory to endoscopic therapy.
Thalidomide, known to have antiangiogenic properties by suppressing VEGF, has been shown in several studies (Table 1) to be a promising treatment option for refractory GIB from GIVM[11-22]. However, only one randomized control trial has been reported by Ge et al[8] in a Chinese population in 2011. In addition, this study has an extensive exclusion criteria, such as comorbidities of cardiac, pulmonary, liver, renal, hematologic, and rheumatologic disorders; history of thromboembolic disease; or patient on antiplatelet agent or anticoagulation. Given that patients with recurrent GIB from GIVM are frequently elderly with several comorbidities, it is unclear whether the results of this study can be extrapolated to other patient populations. Whereas there are several reported case series on the effects of thalidomide on patient with cirrhosis or LVAD[13,20-22], many other studies excluded patients with significant comorbidities (Table 1). We therefore decided to conduct a retrospective study of thalidomide in treating refractory GIB from GIVM in a Western population with significant comorbidities at a tertiary medical center.
| Ref. | No. patients | Patient population | Treatment | Follow-up | Results |
| Heidt et al[18], 2006 | 1 | von-Willebrand's disease type II-a | 100 mg daily | 11 mo | Case report. Recurrent bleed within 2 wk of starting, at 11 months no further bleeding episodes. No side effects. Rebleeding upon cessation and with decreased dose |
| Dabak et al[15], 2007 | 3 | Excluded: HIV positive on treatment. History of seizure disorder. Pregnant or lactating females. Patient with nonmalignant disease (congestive heart failure, uncontrolled infection, etc.). History of noncompliance | Thalidomide 100 mg daily and increased every 2 wk by 100 mg up to 400 mg daily | 250 d | Prospective study. 2/3 patients had responded to thalidomide with decrease transfusion requirements starting at 12 wk of study drug initiation. 1/3 stopped due to lack of response. 1/3 had side effects and dose reduced to 50 mg daily |
| Kamalaporn et al[19], 2009 | 7 | Excluded: Severe medical conditions, such as heart or liver disease | 50 mg daily, increased by 50 mg weekly up to 200 mg daily for 6 mo | 12 mo | Case series. Response to treatment in 3/6 with no blood transfusions at 6 months. 4/7 discontinued thalidomide because of side effects. Upon cessation of thalidomide, of the response group: 1 maintained response with no transfusion for 2 mo, then 1 unit every 4 wk. 1 required 2 u every 3-4 wk, 1 passed from diabetes complications |
| Ge et al[10], 2011 | 55 | Excluded: Cirrhotic or portal gastropathy. Severe comorbidities of cardiac, pulmonary, renal, liver, hematological, rheumatologic, or uncontrollable diabetes mellitus or hypertension. History of severe bilateral peripheral neuropathy or seizure activity, thromboembolic disease, known thalidomide or iron allergy. Treatment with ASA, NSAID, antiplatelet, anticoagulant, or Chinese medications, gingko, echinacea, or immunomodulators. Pregnant or lactating. Undergoing cancer therapy via chemotherapy or radiation | Thalidomide 25 mg four times daily vs ferrous succinate 100 mg four times daily. Both for 4 mo | 39 mo (8-52) | Prospective, randomized, parallel control trial. Effective response rate of decrease bleed > 50% first year of follow-up period in thalidomide 71.4% vs iron supplementation response 3.7% (P < 0.001). Secondary endpoints of rates of bleeding cessation, blood transfusion, overall hospitalization, and hospitalization for bleeding demonstrated thalidomide was more effective. Level of VEGF significantly reduced in thalidomide group (P < 0.001). Minor side effects reported in thalidomide group |
| Garrido Serrano et al[17], 2012 | 19 | Cirrhosis | Thalidomide 200 mg daily for 4 mo | 4 mo | Prospective study. Mean hemoglobin before thalidomide 7 g/dL, after 2 mo 9 g/dL and at end of 4 mo 10 g/dL. Side effects included HE (2/19), sensitive axonal polyneuropathy (1/19) which resolved after withdrawal of thalidomide |
| Ray et al[20], 2014 | 1 | LVAD | 50 mg twice daily | 12 mo | Case report. No further bleeding after starting thalidomide and remained on warfarin. No reported side effects |
| Bond et al[12], 2015 | 1 | Cutaneous T-cell lymphoma | 100 mg daily | 6 mo | Case report. No further bleeding episodes at 6 mo. Side effects of dizziness and unsteadiness |
| Draper et al[23], 2015 | 8 | LVAD | 50 mg twice daily increased by 50 mg weekly up to 200 mg daily | Not included | Case series. No recurrence of bleeding 5/8, reduced bleeding 2/8. Death 1/8 within 1 wk of starting due to sepsis. Side effects in 2/8 |
| Chen et al[14], 2016 | 80 | Excluded: Cirrhotic or portal hypertension gastropathy. Severe cardiac, pulmonary, renal, liver, pancreas, hematological, or rheumatologic comorbidities, uncontrolled diabetes mellitus or hypertension, or renal insufficiency without hemodialysis or peritoneal dialysis. Severe bilateral peripheral neuropathy or seizure, thromboembolic disease. Known thalidomide allergy, treatment with any systemic or oral topical corticosteroids, NSAID, any putative immunomodulators, or antiangiogenic agents. Pregnant or lactating. Alcohol and/or drug abuse. Poor compliance | Thalidomide 25 mg four times a day for 4 mo | 42.6 mo (12-120) | Retrospective study. In first year of follow-up, overall response rate was 77.5% (62/80) with 41.3% (33/80) achieving complete cessation. Overall response rate of 79.5% (62/78). Adverse effects in 60% with serious effects in 31.3% (25/80) |
| Chan et al[13], 2017 | 4 | LVAD | 50 mg daily | 11.4 mo (7-24) | Case series. No further bleeding. When medications stopped, recurrence of bleeding, with restart of medication and cessation of bleeding |
| Duarte et al[16], 2017 | 1 | Glanzmann's thrombasthenia | 50 mg daily, after 15 d increased to 100 mg daily | 6 mo | Case report. Recurrent bleeding at 5 mo, requiring transfusions. Thalidomide suspended at 5 mo. Death due to sepsis and hemorrhage |
| Seng et al[21], 2017 | 11 | LVAD | Thalidomide 50 mg daily | 186 d (3-512 d) | Retrospective study. Resolution of bleeding in 90.9% (10/11). Discontinued in 6 (63.6%) due to resolution of bleeding (n = 4) or side effects (n = 2), with GIB recurring in 4 of these patients. Adverse effects in 5/11 patients including pump thrombosis (n = 2) leading to death. 4/11 died during the study with 1 from continued bleeding and 1 from septic shock |
This is a retrospective study of patients diagnosed with GIVM at the Division of Gastroenterology, Washington University Medical Center/Barnes-Jewish Hospital, from January 2012 to November 2018 who were treated with thalidomide. The institutional review board at Washington University in St. Louis, Missouri approved the study.
Aged ≥ 18 years old; male and post-menopausal females; > 3 episodes of GIB with > 1 episode of refractory bleeding; documented vascular malformation (either GIAD or GAVE) on either upper endoscopy (esophagogastroduodenoscopy) or colonoscopy and if bleeding was not initially found, subsequent balloon enteroscopy or capsule endoscopy; and treated with thalidomide for at least 3 months. Refractory bleeding was defined as recurrent bleeding requiring > 2 transfusions after failing 2 treatments with endoscopic therapy using APC or medical therapies, such as octreotide, estrogen, or aminocaproic acid. Exclusion criteria: Patients with other causes of GIB, patients with allergy to thalidomide, women who were pregnant or lactating, and pre-menopausal women.
All of the patients included in this study were prescribed thalidomide (Thalomid®) by a single certified provider (Chen CH) according to the Thalomid Risk Evaluation and Mitigation Strategy (REMS) program of Celgene (NJ, United States). Patients were started on thalidomide for more than 3 episodes of GIB requiring blood transfusion with recurrent GIB after failing at least two treatments with endoscopic therapy or medical therapy. Patients were started on thalidomide 50 mg twice daily or 100 mg once daily. This initial dose was increased or decreased depending on patient’s response and ability to tolerate the medication. During the treatment period, patients were monitored by phone every 4 wk and in the outpatient clinic every 3 to 6 mo. Complete blood count and comprehensive metabolic panel were monitored every 3 to 6 mo.
In the event of an adverse side effect, thalidomide was temporarily or permanently discontinued depending on the severity of the side effect and the patient’s tolerability. During treatment with thalidomide, adjunct therapy such as blood transfusions or repeat endoscopic treatment were performed as necessary at the discretion of treating physicians. Transfusion was given to patients when hemoglobin level fell below 7.0 g/dL. Patients were continued on antiplatelet or anticoagulation or iron supplementation if they were taking it prior to initiation of thalidomide.
Data collected from electronic medical records included patient’s demographics, medical history, comorbidities, medications, number of hospitalizations, endoscopic treatment, and number of blood transfusions.
The primary endpoint was to assess the recurrence of GIB 6 mo after the initiation of thalidomide. The secondary endpoints were the number of hospitalizations, blood transfusion requirements, and endoscopic treatments. A bleeding recurrence was defined as a GIB episode with hemoglobin level less than 7.0 g/dL resulting in a blood transfusion. All bleeding episodes of the patients studied were due to GIVM. Patients were encouraged to keep in close contact with patient care coordinators to update the team on their progress, possible side effects from thalidomide, and any bleeding episodes requiring blood transfusions or hospitalizations that occurred in between office visits.
A total of 15 patients were included in this study. Table 2 details the demographic and clinical characteristics of patients included in this study. Mean age was 69 (range: 58-83) years old. There were 6 women (40%) and 9 men (60%). Eleven patients were Caucasian (73.3%) and four patients were African American (26.7%). Body mass index median was 28.6 (range: 21-48). All patients in this study had multiple comorbidities. Six patients had cirrhosis (n = 5) or non-cirrhotic portal hypertension; Child-Pugh class A (n = 2), class B (n = 3). Three had end stage renal disease (ESRD) on hemodialysis, 3 had chronic kidney disease, stage 3 (n = 2), stage 5 (n = 1). Three had a LVAD, 10 had hypertension, 5 had diabetes, 1 had myelodysplastic syndrome (MDS) and non-Hodgkin’s lymphoma, 1 had polyclonal gammopathy, 2 had aortic stenosis, and 1 had hereditary hemorrhagic telangiectasias.
| Patient | Age | Sex | Comorbidities | Cause of GIB | Prior failed treatments | Iron supplementation | Comments |
| 1 | 58 | M | LVAD | GIAD | Octreotide | ||
| 2 | 67 | F | ESRD | GIAD | Sodium ferric gluconate | Aspirin | |
| 3 | 70 | M | LVAD | GIAD | Octreotide | Ferrous sulfate | Warfarin |
| 4 | 61 | F | Cirrhosis | GIAD | Ferrous sulfate | ||
| 5 | 81 | M | LVAD | GIAD | Octreotide | Ferrous sulfate | Aspirin, warfarin |
| 6 | 83 | M | CAD, AS, AAA, CVA | GIAD | Octreotide, estrogen | Polysaccharide iron | Aspirin, cilostazol, plavix |
| 7 | 72 | M | Hepatoportal sclerosis | GAVE | |||
| 8 | 69 | F | Polyclonal gammopathy | GIAD | |||
| 9 | 70 | F | ESRD, HHT | GIAD | Octreotide | Ferrous sulfate | |
| 10 | 62 | M | Cirrhosis | GAVE | Ferrous fumarate | ||
| 11 | 58 | M | Cirrhosis, CHF, ESRD | GAVE | Octreotide | Ferrous sulfate | |
| 12 | 64 | F | Cirrhosis, MDS, NHL | GAVE | |||
| 13 | 80 | F | AS | GIAD | |||
| 14 | 74 | M | NSCLC, AFIB, MDS | GIAD | Lost to follow-up | ||
| 15 | 68 | M | AFIB, CHF, Cirrhosis | GAVE | Aspirin, warfarin, lost to follow-up |
The cause of GIB was GIAD in 10 patients (66.6%) and GAVE in 5 patients (33.3%). Prior to treatment with thalidomide, 6 patients were previously treated with octreotide, 1 treated with estrogen, and 1 treated with aminocaproic acid. All patients received at least two treatments of APC before starting thalidomide, except for one whose source of bleeding was not reachable via endoscopy. All patients prior to the initiation of thalidomide had anemia with average hemoglobin level of 7.9 g/dL. Five patients were on iron supplement when referred to our care and 3 patients with iron deficiency were not on iron supplement. Eight patients continued with iron supplementation after initiation of thalidomide treatment. Five patients remained on antiplatelet or anticoagulation such as aspirin, warfarin, clopidogrel, or cilostazol. Two patients discontinued thalidomide: One due to cost and another due to drug interaction with methadone, after being treated with thalidomide for 17 mo and 3 mo respectively. Two other patients were lost to follow up.
During the one year prior to starting thalidomide, of the 13 patients who remained in the study, the mean number of hospital admissions for GIB was 5.46 (range, 1-20 admissions), the mean number of packed red blood cell (PRBC) transfusions was 31 units (range, 8-99 units) and the mean number of endoscopic treatments was 4.54 (range, 0-12 endoscopic treatments).
Thalidomide was started at either 50 mg twice daily or 100 mg once daily. Ultimately the dose of thalidomide ranged from 50 mg to 200 mg daily, titrating to patients’ response and tolerability. The average duration of treatment on thalidomide was 28 mo (range, 3-45 mo).
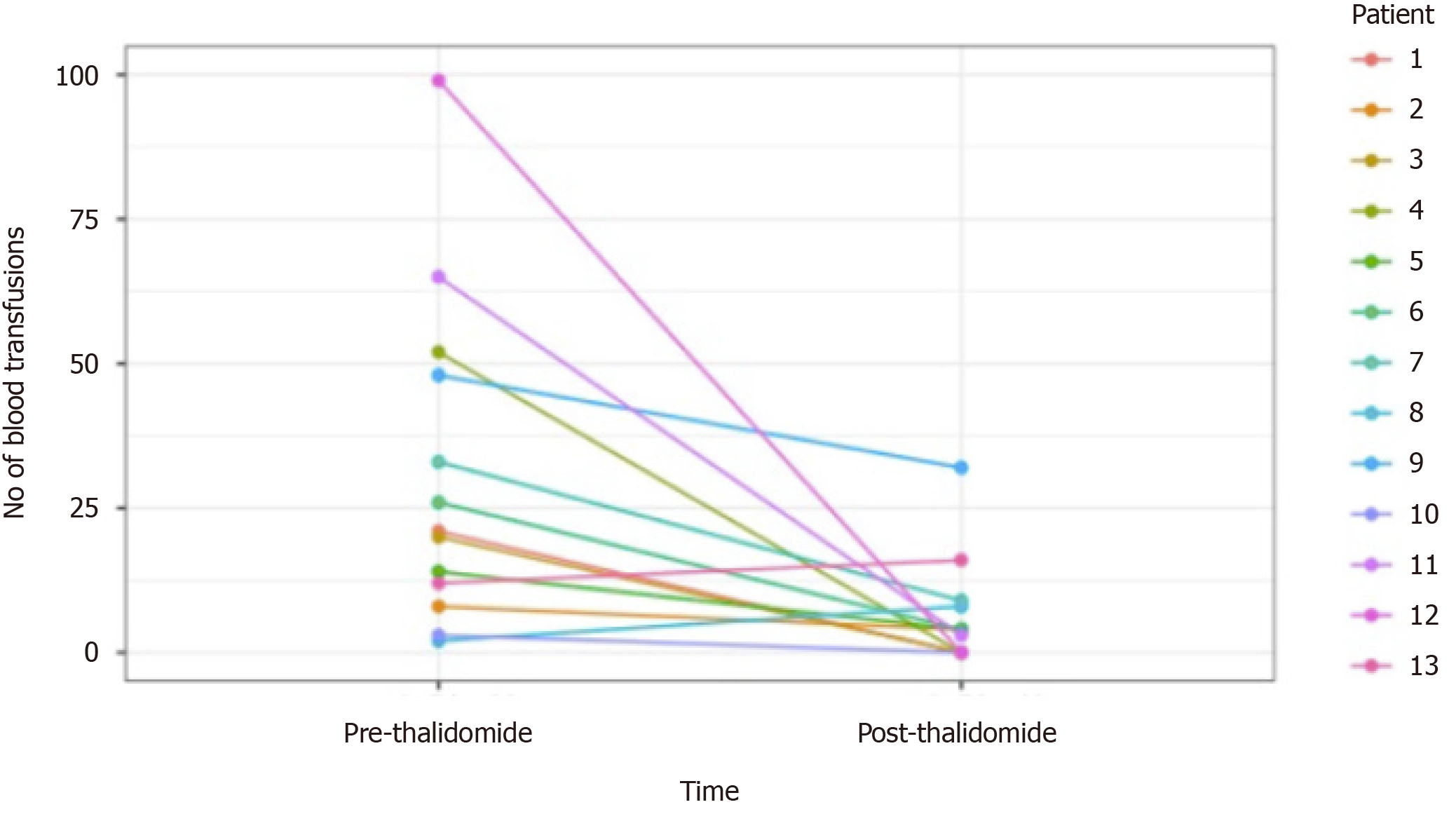
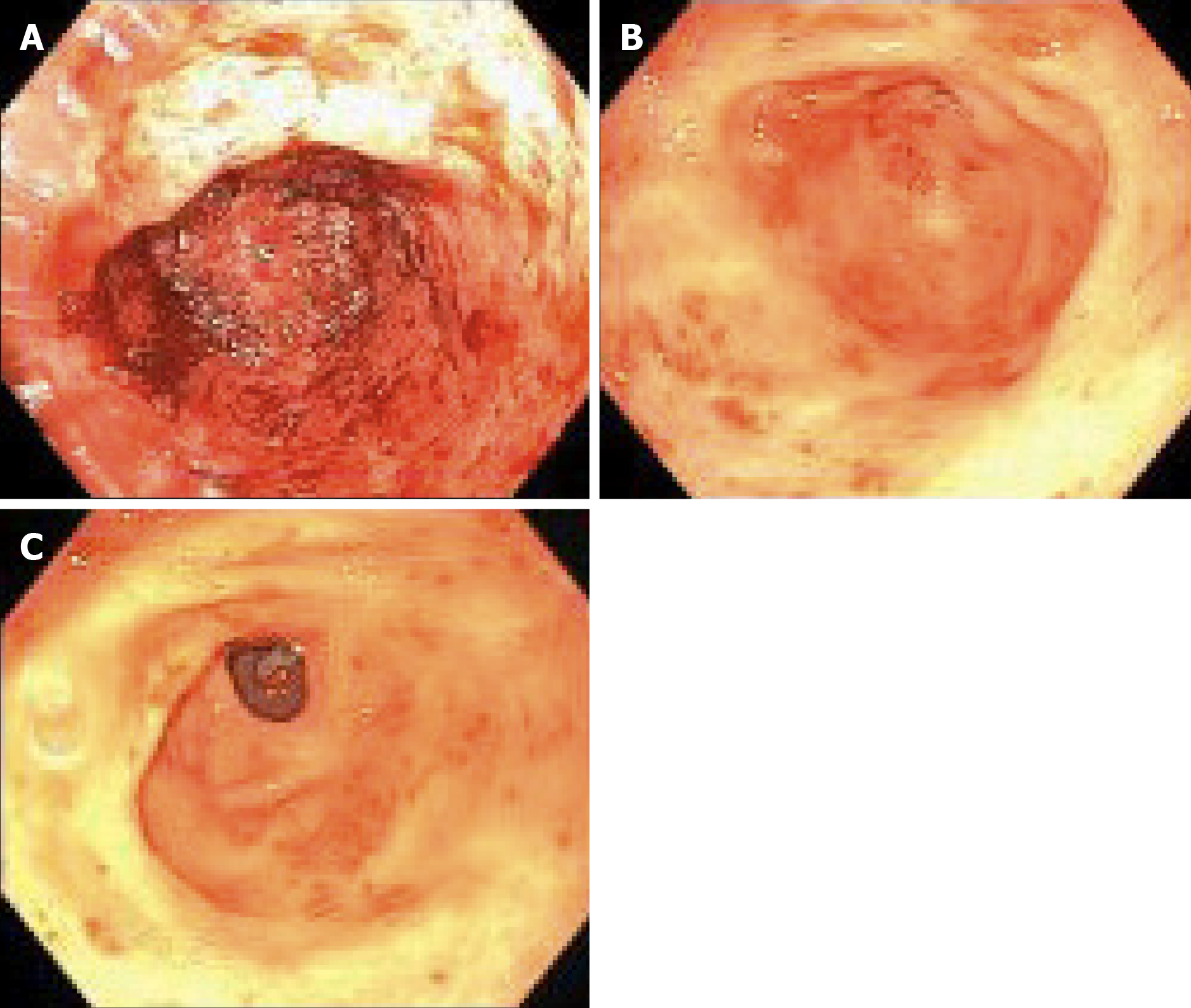
Of the 13 patients followed (range 3-45 mo), 9 patients had GIAD and 4 patients had GAVE. GIAD were located in the gastric fundus (n = 1), duodenum (n = 3), jejunum (n = 5), ileum (n = 1), and hepatic flexure of colon (n = 1). After initiation of thalidomide treatment for 6 mo, 5 patients (38.5%) met our primary endpoint with no recurrent GIB episodes during follow-up (Figure 1). Of these 5 patients, 2 had an LVAD and GIAD, 2 had cirrhosis and GAVE, and 1 had cirrhosis and GIAD. Another 6 patients (46.2%) had both marked reduction in transfusion requirements and hospitalizations for GIB (Figure 1 and Table 3). Two patients had increased transfusion after treatment with thalidomide for 3 and 30 mo. The requirement of endoscopic treatments decreased in 11 patients (84.6%) after the initiation of treatment. Of the 11 patients who responded favorably to treatment, the effect of thalidomide could be observed after 3 mo of treatment. Significant reduction of GIVM could be seen during follow-up endoscopies, as demonstrated in a patient with GAVE who underwent esophagogastroduo-denoscopy 3 and 8 mo after treatment with thalidomide (Figure 2).
| Patient | Duration of treatment (mo) | Recurrence of bleeding after 6 mo | Number of hospitalizations due to GIB | Units RBC transfused | Number of endoscopic treatments (APC) | Comments | |||
| 1 yr before thalidomide | After starting thalidomide | 1 yr before thalidomide | After starting thalidomide | 1 yr before thalidomide | After starting thalidomide | ||||
| 1 | 6 | No | 2 | 0 | 21 | 0 | 2 | 0 | |
| 2 | 19 | Yes | 4 | 1 | 8 | 12 | 3 | 1 | Side effect of fatigue and dizziness |
| 3 | 45 | No | 2 | 0 | 20 | 0 | 1 | 0 | Side effect of neuropathy |
| 4 | 14 | No | 1 | 0 | Weekly transfusion | 0 | 6 | 0 | Side effect of fatigue and constipation. Death |
| 5 | 44 | Yes | 5 | 1 | 14 | 4 | 4 | 1 | Side effect of neuropathy. Death, Sepsis |
| 6 | 17 | Yes | 6 | 5 | Biweekly transfusion | 4 | 3 | 2 | Stopped due to cost. Death |
| 7 | 24 | Yes | 9 | 3 | 33 | 9 | 4 | 10 | Death |
| 8 | 30 | Yes | 1 | 2 | 2 | 8 | 2 | 1 | Side effect of fatigue |
| 9 | 28 | Yes | 17 | 8 | 48 | 32 | 12 | 1 | Death |
| 10 | 3 | NA | 1 | 0 | 3 | 0 | 1 | 0 | Stopped due to drug interaction |
| 11 | 25 | No | 20 | 1 | 65 | 3 | 12 | 1 | Death, Sepsis |
| 12 | 6 | No | 2 | 0 | 99 | 0 | 9 | 4 | Side effect of neutropenia |
| 13 | 3 | NA | 1 | 1 | 12 | 16 | 1 | 1 | |
All 4 patients who remained on anticoagulation or antiplatelet therapy, including 2 with an LVAD, had a significant decrease in GIB as demonstrated by a decreased transfusion requirements and hospitalizations. Additionally, all 4 patients with cirrhosis had a marked decrease in hospitalizations, blood transfusions, and endoscopic treatments. The 3 patients with ESRD on dialysis and 3 patients with chronic kidney disease all showed improvement in transfusion requirements after treatment.
Reported adverse reactions were documented in 6 of the 13 patients (46%), including fatigue (n = 3), neuropathy (n = 2), constipation (n = 1), and dizziness (n = 1). Worsening neutropenia was observed in a patient with concurrent MDS, with stabilization of white blood cell counts after a dose reduction of thalidomide from 100 mg to 50 mg daily. GIB did not recur after this dose reduction. Six patients died during the follow-up period of this study. Five patients died while on thalidomide due to causes unrelated to recurrent GIB and one patient died from unknown causes after discontinuing thalidomide due to cost. All patients who died while taking thalidomide had been treated for over 1 year. Of the 5 patients who died while taking thalidomide, 2 died from complications due to sepsis, 1 sustained a traumatic fall and passed away in hospice, and 2 died from unknown causes.
This retrospective study aimed to examine the effect of thalidomide on refractory GIB due to GIVM. Our results demonstrated that thalidomide appears to be an effective medical therapy for this very challenging patient population. Five out of 13 patients (38.5%) followed in our study had no recurrent GIB after treatment with thalidomide, while overall 11 out of 13 patients (86.4%) had decrease in recurrence of GIB, hospitalizations, blood transfusions and endoscopic therapies.
Previous studies and case reports have reported similar results with thalidomide in treating recurrent GIB from GIVM[8,10-22]. Whereas the only randomized controlled trial so far by Ge et al[10] from China found a response rate of 71.4%, patients with cardiac, pulmonary, hepatic, renal, and other comorbidities were excluded from the study. In a follow-up retrospective study by the same group with 80 patients, the response rate to thalidomide in patients with or without comorbidities was 76.7% and 78% respectively, although this study again excluded patients with severe comorbidities such as cirrhosis and severe cardiac and renal conditions[14]. Therefore, it is unclear whether these results could be applied to other patient populations with significant comorbidities. Three previous case series of patients with an LVAD[13] and one case study of patients with liver cirrhosis[17] found thalidomide effective in treating refractory GIB patients with these comorbidities. The response rate in our study was 84.6%, comparable to other previous studies. The patients in our study represent the most challenging recurrent GIB patients we encounter in a tertiary referral medical center, as evidenced by their multiple comorbidities, and the average number of admissions (5.46) and transfusions (31 units of PRBC) one year before the initiation of thalidomide. Our study demonstrated that thalidomide remains an effective treatment for refractory GIB from GIVM in a Western population, including patients with severe comorbidities such as LVAD, cirrhosis, and ESRD on dialysis.
Anticoagulation or antiplatelet agents are frequently discontinued in the setting of GIB. However, this increases the risk of thromboembolism especially in patients with significant cardiovascular comorbidities and patients with LVAD. Four of our patients continued their anticoagulation or antiplatelet agents such as warfarin, aspirin, clopidogrel, or cilostazol, and still responded favorably to thalidomide. Our results suggest that it may not be necessary to discontinue anticoagulation or antiplatelet agents in this group of patients with severe multiple comorbidities while on thalidomide. One patient with severe GAVE and platelet count < 30000/mL due to MDS received 99 units of PRBC in the one year before thalidomide treatment, and required no transfusion after treatment. More data is needed to determine whether correcting thrombocytopenia is necessary when treating GIVM with thalidomide.
Thalidomide is thought to inhibit angiogenesis by suppressing VEGF. In our study, the effects of thalidomide became apparent after about 3 mo of treatment, consistent with the observation of previous studies[15,17,19]. The interval between the initiation of thalidomide and the decrease of GIB likely reflects the time it takes to change the balance between anti- and pro- angiogenesis factors in the existing GIVM and suppressing new GIVM formation. The effectiveness of thalidomide in treating GIB from GIVM as shown in this and other studies suggests that we could potentially explore other angiogenesis inhibitors to treat GIVM.
The dose of thalidomide in previous studies ranged from 50 mg to 400 mg per day. Dosing and efficacy could possibly be related to a patient’s body mass index (BMI), as was suggested by Seng et al[20] where BMI median was 18. The initial dose of thalidomide in their study was 50 mg daily for all patients, whereas other studies typically used at least 100 mg daily[16,20]. In our study, the dose of thalidomide was started at 100 mg daily, with the final dosage ranged from 50 to 200 mg daily. The median BMI of our patients was 28. Seven of our patients improved on thalidomide 100 mg daily, whereas two patients did not see improvement of bleeding until the dose of thalidomide was increased to 150 and 200 mg daily, with a BMI of 28 and 48 respectively. Two non-responders in our study were treated with 200 mg daily with a BMI of 30, and 100 mg daily with BMI of 24. We do not see a clear association of dose, BMI, and effectiveness based on our data. Further study is needed to elucidate the relationship between the response to thalidomide and BMI.
The common side effects of thalidomide include drowsiness, fatigue, dizziness, neuropathy, skin rash, and constipation. The most common side effect reported in our study was fatigue (n = 3). Fatigue and dizziness were reported in one patient who mistakenly took double dose of thalidomide, and resolved once she went back to the prescribed dose. Neutropenia (n = 1) occurred in a patient with MDS, and resolved upon reducing the dose of thalidomide to 50 mg daily and without recurrence of GIB. Neuropathy was described in 2 patients, one of which had a history of parkinsonism and another with a history of diabetes. No thromboembolism was observed in our study, although the reported risk of thromboembolism associated with thalidomide is mainly seen in patients with multiple myeloma on chemotherapy. Five patients died while on thalidomide and one patient died after discontinuation of thalidomide due to cost. The cause of death does not appear to be associated with thalidomide (sepsis 2, fall 1) in 3 patients. The cause of death in 2 patients was unknown and therefore unclear if it is related to thalidomide.
The major limitations of this study are the limitations inherent with a retrospective study, such that we were not able to control for variables like the degree of IDA or whether patients were taking iron supplement. Another limitation is our small sample size (n = 15). Most of our patients with refractory GIB were elderly with limited income. Many patients could not receive treatment with thalidomide due to cost or lack of insurance coverage. Even more patients declined treatment with thalidomide due to concern of potential side effects in the context of their multiple comorbidities after reading the patient medical information from REMS. Nevertheless, our sample size is the second largest from the Western World in the literature. In addition, our primary and secondary outcomes were objective measures and less susceptible to reporting bias. With more data published on thalidomide in recurrent GIB, we may see improvement in its coverage or patients’ acceptance.
In conclusion, the treatment of patients with refractory GIB due to GIVM remains a daunting task with limited options. Our study suggests that thalidomide appears to be an effective treatment for refractory GIB due to GIAD or GAVE in a Western population with significant comorbidities such as LVAD, portal hypertension, and ESRD.
Gastrointestinal vascular malformations (GIVM) consist of several types such as gastrointestinal angiodysplasias (GIAD) and gastric antral vascular ectasias (GAVE). GIAD are abnormal, dilated, tortuous vessels within the mucosal and submucosal layers and are the most common GIVM seen throughout the gastrointestinal tract. GAVE is generally found in patients with chronic illnesses, such as liver disease, connective tissue diseases, and chronic renal failure. Refractory gastrointestinal bleeding (GIB) secondary to GIVM remains challenging to treat when endoscopic therapy fails. Endoscopic therapy with argon plasma coagulation (APC) is the mainstay of treatment for GIVM. However, GIVM refractory to endoscopic treatment is common and medications such as octreotide, tranexamic acid, hormonal therapy such as estrogen-progesterone, and thalidomide have all been studied in the management of recurrent GIB refractory to endoscopic therapy. Thalidomide, known to have antiangiogenic properties by suppressing VEGF, has been suggested recently as a treatment option for refractory GIB.
Only one randomized control trial demonstrating the efficacy of thalidomide for treating refractory GIB due to GIVM has been published in 2011 in a Chinese population. However, the study had extensive exclusion criteria and it is unclear whether the results of the study can be extrapolated to other patient populations. We therefore decided to conduct a retrospective study of thalidomide in treating refractory GIB from GIVM in a Western population with significant comorbidities at a tertiary medical center.
To evaluate thalidomide as a treatment option for patients who suffer from refractory GIB due to GIVM.
Single center, IRB approved, retrospective review of electronic medical records from January 2012 to November 2018. Patients age > 18 years old, who had > 3 episodes of GIB refractory to medical or endoscopic therapy and documented to be due to GIVM, and who had been treated with thalidomide for at least 3 months were included. Refractory bleeding was defined as recurrent bleeding requiring > 2 transfusions after failing 2 treatments with endoscopic therapy using APC or medical therapies, such as octreotide, estrogen, or aminocaproic acid. The primary endpoint was recurrence of GIB 6 mo after initiation of thalidomide. The secondary endpoints were the number of hospitalizations, blood transfusion requirements, and endoscopic treatments.
Fifteen patients were included in the study, all with significant cardiac, hepatic, or renal comorbidities. The cause of GIB was GIAD in 10 patients and GAVE in 5 patients. Two patients were lost to follow up. Of the 13 patients followed, 38.5% (n = 5) had no recurrent GIB or transfusion requirement after treatment with thalidomide. Furthermore, 84.6% (n = 11) of patients had a reduction in transfusion requirements and hospitalizations for GIB. Thalidomide was discontinued in 2 patients due to cost (n = 1) and medication interaction (n = 1). Reported adverse reactions included fatigue (n = 3), neuropathy (n = 2), dizziness (n = 1), and constipation (n = 1). Six patients died during follow up due to unknown cause (n = 4) and sepsis (n = 2).
Our results demonstrated that thalidomide appears to be an effective medical therapy for refractory GIB due to GIVM. The response rate in this study was 84.6%, comparable to other previous studies. The patients in our study represent the most challenging recurrent GIB patients in a tertiary referral medical center, as evidenced by their multiple comorbidities, and the average number of admissions (5.5) and transfusions (31 units of PRBC) one year before the initiation of thalidomide. Our study demonstrated that thalidomide remains an effective treatment for refractory GIB from GIVM in a Western population, including patients with severe comorbidities such as left ventricular assist device, cirrhosis, and end-stage renal disease on dialysis.
Based on the results of this study, future research should include prospective randomized control trial with a larger patient population so that we can examine the effect of thalidomide on each comorbidity with sufficient power.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Ho CM, Lee J, Leontiadis GI S-Editor: Yan JP L-Editor: A E-Editor: Wang LL
| 1. | Junquera F, Saperas E, de Torres I, Vidal MT, Malagelada JR. Increased expression of angiogenic factors in human colonic angiodysplasia. Am J Gastroenterol. 1999;94:1070-1076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Feng N, Chen H, Fu S, Bian Z, Lin X, Yang L, Gao Y, Fang J, Ge Z. HIF-1α and HIF-2α induced angiogenesis in gastrointestinal vascular malformation and reversed by thalidomide. Sci Rep. 2016;6:27280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Nardone G, Compare D, Martino A, Rocco A. Pharmacological treatment of gastrointestinal bleeding due to angiodysplasias: A position paper of the Italian Society of Gastroenterology (SIGE). Dig Liver Dis. 2018;50:542-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Draper KV, Huang RJ, Gerson LB. GI bleeding in patients with continuous-flow left ventricular assist devices: a systematic review and meta-analysis. Gastrointest Endosc. 2014;80:435-446.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 5. | Chalasani N, Cotsonis G, Wilcox CM. Upper gastrointestinal bleeding in patients with chronic renal failure: role of vascular ectasia. Am J Gastroenterol. 1996;91:2329-2332. [PubMed] [Cited in This Article: ] |
| 6. | Alkhormi AM, Memon MY, Alqarawi A. Gastric Antral Vascular Ectasia: A Case Report and Literature Review. J Transl Int Med. 2018;6:47-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Vuddanda V, Jazayeri MA, Turagam MK, Lavu M, Parikh V, Atkins D, Bommana S, Yeruva MR, Di Biase L, Cheng J, Swarup V, Gopinathannair R, Olyaee M, Ivaturi V, Natale A, Lakkireddy D. Systemic Octreotide Therapy in Prevention of Gastrointestinal Bleeds Related to Arteriovenous Malformations and Obscure Etiology in Atrial Fibrillation. JACC Clin Electrophysiol. 2017;3:1390-1399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Ge ZZ, Chen HM, Gao YJ, Liu WZ, Xu CH, Tan HH, Chen HY, Wei W, Fang JY, Xiao SD. Efficacy of thalidomide for refractory gastrointestinal bleeding from vascular malformation. Gastroenterology. 2011;141:1629-37.e1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 9. | Grooteman KV, van Geenen EJM, Drenth JPH. Tranexamic acid in treatment-resistant chronic transfusion-dependent gastrointestinal angiodysplasia bleeding. BMJ Case Rep. 2017;2017:bcr2017221832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Tran A, Villeneuve JP, Bilodeau M, Willems B, Marleau D, Fenyves D, Parent R, Pomier-Layrargues G. Treatment of chronic bleeding from gastric antral vascular ectasia (GAVE) with estrogen-progesterone in cirrhotic patients: an open pilot study. Am J Gastroenterol. 1999;94:2909-2911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Bauditz J. Effective treatment of gastrointestinal bleeding with thalidomide--Chances and limitations. World J Gastroenterol. 2016;22:3158-3164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 26] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Bond A, Ahmed W. Thalidomide for the treatment of angiodysplasia in a patient with acute upper.gastrointestinal haemorrhage. BMJ Case Rep. 2016;2016:bcr2015213522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Chan LL, Lim CP, Lim CH, Tan TE, Sim D, Sivathasan C. Novel Use of Thalidomide in Recurrent Gastrointestinal Tract Bleeding in Patients with Left Ventricular Assist Devices: A Case Series. Heart Lung Circ. 2017;26:1101-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Chen H, Fu S, Feng N, Chen H, Gao Y, Zhao Y, Xue H, Zhang Y, Li X, Dai J, Fang J, Ge Z. Bleeding recurrence in patients with gastrointestinal vascular malformation after thalidomide. Medicine (Baltimore). 2016;95:e4606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Dabak V, Kuriakose P, Kamboj G, Shurafa M. A pilot study of thalidomide in recurrent GI bleeding due to angiodysplasias. Dig Dis Sci. 2008;53:1632-1635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Duarte BKL, de Souza SM, Costa-Lima C, Medina SS, Ozelo MC. Thalidomide for the Treatment of Gastrointestinal Bleeding Due to Angiodysplasia in a Patient with Glanzmann's Thrombasthenia. Hematol Rep. 2017;9:6961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Garrido Serrano A, León R, Sayago M, Márquez JL. Thalidomide treatment in cirrhotic patients with severe anemia secondary to vascular malformations. Dig Dis Sci. 2012;57:1112-1113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Heidt J, Langers AM, van der Meer FJ, Brouwer RE. Thalidomide as treatment for digestive tract angiodysplasias. Neth J Med. 2006;64:425-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Kamalaporn P, Saravanan R, Cirocco M, May G, Kortan P, Kandel G, Marcon N. Thalidomide for the treatment of chronic gastrointestinal bleeding from angiodysplasias: a case series. Eur J Gastroenterol Hepatol. 2009;21:1347-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Ray R, Kale PP, Ha R, Banerjee D. Treatment of left ventricular assist device-associated arteriovenous malformations with thalidomide. ASAIO J. 2014;60:482-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Seng BJJ, Teo LLY, Chan LL, Sim DKL, Kerk KL, Soon JL, Tan TE, Sivathasan C, Lim CP. Novel use of low-dose thalidomide in refractory gastrointestinal bleeding in left ventricular assist device patients. Int J Artif Organs. 2017;40:636-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Draper K, Kale P, Martin B, Kelly Cordero R, Ha R, Banerjee D. Thalidomide for treatment of gastrointestinal angiodysplasia in patients with left ventricular assist devices: case series and treatment protocol. J Heart Lung Transplant. 2015;34:132-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 23. | Carrier M, Le Gal G, Tay J, Wu C, Lee AY. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysis. J Thromb Haemost. 2011;9:653-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |