Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4876
Peer-review started: June 6, 2020
First decision: September 13, 2020
Revised: September 15, 2020
Accepted: September 25, 2020
Article in press: September 25, 2020
Published online: October 26, 2020
Paliperidone palmitate is a once-monthly injectable, atypical antipsychotic. To our knowledge, there has been only one report of paliperidone palmitate-induced angioedema presenting with acute laryngeal edema with subsequent respiratory arrest. Here, we present a case report of paliperidone palmitate-induced angioedema with a relatively mild clinical presentation compared with the previously reported case, and the patient’s condition was not complicated by life-threatening anaphylaxis.
A 79-year-old female, who had a major neurocognitive disorder due to Alzheimer’s disease with behavioral disturbances. Paliperidone palmitate was off-label used to control her aggression, irritability, and psychosis. After induction doses (150 mg and 100 mg intramuscularly, given 1 wk apart), she developed intermittent swelling of the face, eyelids, and lips on day 17 after the initial dose, and the edema was explicitly seen on day 20. The diagnosis was paliperidone palmitate-induced angioedema. The monthly injection dose was discontinued on day 33 after the initial dose. The angioedema was subsequently alleviated, and it had completely resolved by day 40 after the initial dose.
Paliperidone palmitate-induced angioedema is a rare condition and can present with a mild, intermittent facial edema, which may be overlooked in clinical practice.
Core Tip: Paliperidone palmitate-induced angioedema can present with insidious onset, intermittent, facial edema which may be overlooked. Previous reports show that paliperidone-induced angioedema, which may cause life-threatening laryngeal edema, may be dose-related. This report raises awareness of this rare, adverse effect, angioedema. Close monitoring of patients should be performed when they are administered paliperidone palmitate, especially psychiatric patients who are unable to detect their illness.
- Citation: Srifuengfung M, Sukakul T, Liangcheep C, Viravan N. Paliperidone palmitate-induced facial angioedema: A case report. World J Clin Cases 2020; 8(20): 4876-4882
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4876.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4876
Paliperidone palmitate is a long-acting, atypical antipsychotic medication that is administered via monthly intramuscular (IM) injections. It is used to treat the psychotic symptoms of schizophrenia and delay the time to relapse[1,2]. In addition, the drug can be effectively used in patients with poor medication compliance to reduce recurrence and hospitalization rates[3]. Moreover, in patients with major neurocognitive disorders, it has been used off-label to control neuropsychiatric symptoms, including delusion, hallucination, agitation, and aggression. Paliperidone palmitate can also prevent relapse of schizophrenia as effective as fluphenazine decanoate while carrying a lower risk of tardive dyskinesia and anticholinergics use[4].
Angioedema refers to deep dermal, subcutaneous, and submucosal edemas of tissue caused by the release of vasoactive mediators. Medication-induced angioedema can lead to mortality if laryngeal edema occurs[5]. Angioedema has been reported to be associated with antipsychotics, e.g., chlorpromazine[6], haloperidol, iloperidone[7], risperidone[8], quetiapine, olanzapine, clozapine[9], and ziprasidone[10]. To our knowledge, there are only two previous reports of paliperidone-induced angioedema. The first case involved facial angioedema arising from dose-dependent oral paliperidone[11], while the second case concerned acute laryngeal edema with respiratory arrest resulting from a paliperidone palmitate injection[12]. The current report presents a rare case of paliperidone palmitate-induced angioedema that presented with intermittent facial edema. The previously reported cases of paliperidone-induced angioedema are also reviewed and discussed.
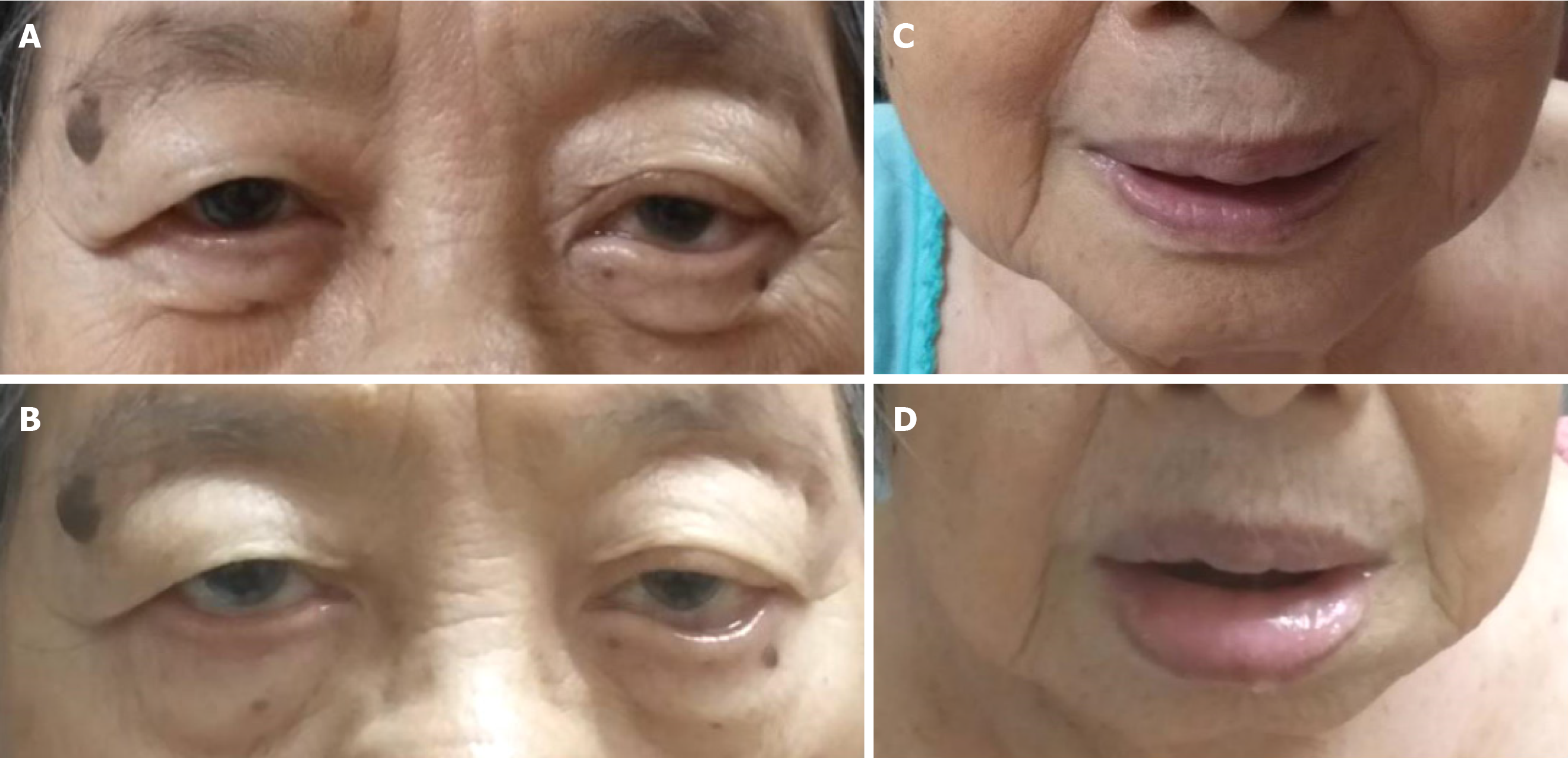
Intermittent facial edema at day 17 after an initial dose of paliperidone palmitate, administered by intramuscular injection.
A 79-year-old Thai woman was admitted to a psychiatric ward due to uncontrollable aggressive behaviors and psychotic symptoms. A retired teacher living with two daughters, she had started having memory problems and episodes of irritable mood two years prior to the admission. Moreover, she had been diagnosed with a major neurocognitive disorder due to Alzheimer’s disease one year before the admission, and she was treated with donepezil (5 mg/d). Two weeks before admission, she had developed progressive irritable mood, aggressive behaviors, nihilistic delusions, visual and auditory hallucinations, and agitation, which prompted her daughters to take her to the outpatient clinic. Her primary doctor believed that the patient’s psychiatric symptoms were explained by the progression of Alzheimer’s disease rather than donepezil-induced hallucination because donepezil had been constantly given at low dose (5 mg/d) for one year. Initially, oral risperidone, titrated to 2 mg/d, was administered for five days, but it was unable to control her behaviors. The medication was then switched to oral quetiapine, titrated to 400 mg/d, for five days; unfortunately, it too failed to control her aggressive behaviors and psychosis. She was therefore admitted.
During admission, oral quetiapine was titrated to 600 mg/d but was unable to control her violent behaviors. Quetiapine was switched to oral paliperidone and was titrated from 3 to 6 mg/d over four days. The patient responded well to paliperidone, and the psychotic and aggressive behaviors were markedly ameliorated. However, she still had a low tendency to comply with oral paliperidone; consequently, after nine days, the drug was switched to paliperidone palmitate. An induction dosage was given in the form of an initial dose of 150 mg IM, followed by 100 mg IM the next week. The patient was discharged from the hospital with no oral antipsychotic.
At home, 17 d after receiving the initial dose of paliperidone palmitate, the patient started to show mild facial swelling with symmetrical eyelids and lip edema without itchiness (Figure 1). The symptoms usually lasted two to three hours and disappeared with no treatment. A few days later, on day 20 after the initial dose, the edema became worse and prominent. There were no other manifestations that indicated anaphylaxis, such as breathlessness, signs of hypotension, nausea, and vomiting. No other specified rash was observed. The patient denied taking any other nonprescription medications, or new kinds of food or herbs.
The patient had an underlying, major neurocognitive disorder due to Alzheimer’s disease; type 2 diabetes mellitus; dyslipidemia; and hypertension. Her current medications were donepezil (5 mg/d), metformin (500 mg/d), simvastatin (500 mg/d), felodipine (5 mg/d), and atenolol (25 mg/d). All medications had been taken orally and regularly at a stable dose for more than a year preceding the edema.
The patient had a history of aspirin allergy of unknown presentation since childhood. She had never had facial edema caused by food. The patient also had no relatives who had reported being allergic to medications or who had had angioedema.
The patient visited the outpatient clinic 33 d after receiving the initial dose of paliperidone palmitate. Her vital signs were stable: Body temperature, 37.0 °C; Heart rate, 68 bpm; Respiratory rate, 18 breaths per minute; Blood pressure, 122/60 mmHg; Oxygen saturation in room air, 98%. There was no edema of the face or extremities, nor rash or skin discoloration. Both lungs were clear on auscultation, and all other systems were normal.
The patient’s serum renal, hepatic, and thyroid functions, including electrolytes, were normal. Naranjo’s algorithm score[13] was 6 (probable adverse drug reaction).
Based on the patient’s history and the Naranjo score, the final diagnosis was paliperidone palmitate-induced angioedema.
On day 33 after the initial paliperidone palmitate dose had been given, the patient visited the outpatient clinic. Her scheduled monthly paliperidone palmitate dose was stopped and replaced with oral paliperidone (3 mg/d). Antihistamine, corticosteroid, and epinephrine were not given to the patient.
After the injectable paliperidone palmitate was stopped, the patient’s facial edema reduced and had completely resolved by day 40 after the initial dose of paliperidone palmitate. The oral paliperidone was titrated to 6 mg/d and was continued for two months before being tapered off due to clinical stability. No recurrent facial edema or other allergic symptoms were observed during subsequent, routine, follow-up visits.
Paliperidone palmitate is a long-acting, injectable formula of the atypical antipsychotic, paliperidone. Approved by the Unites States Food and Drug Administration for the treatment of schizophrenia and schizoaffective disorders, it has been in use since 2009. It is given by intramuscular injection, with the first two induction doses (150 mg and 100 mg) being given one week apart, followed by a single, monthly, maintenance dose of 25–150 mg[14]. Its clinical effects can be seen on day 8 after the initial dose. Paliperidone palmitate reaches its peak plasma level on days 12-13, and its serum half-life is 25-49 d[15,16].
Angioedema refers to deep dermal, subcutaneous, and submucosal edemas of the tissue. The edema is non-pitting and tends to appear in the lax-skin area. Angioedema often involves the face, lips, and tongue, and it can be fatal if the larynx or pharynx is involved. The pathogenesis of medication-induced angioedema can be either an immunological reaction (such as an IgE-mediated, complement activation), or a non-immunological reaction (for example, interference with arachidonic acid metabolism, kinin-dependent mechanisms, or direct mast cell degranulation)[5]. To date, the mechanism of antipsychotic-induced angioedema remains unclear; the available evidence indicates that it could involve either immunological[17] or non-immunological reactions[18].
To our knowledge, there is only one reported case of angioedema resulting from paliperidone palmitate injections. The case concerned a 30-year-old male with schizophrenia from Greece[12] (Table 1). He developed acute laryngeal edema and respiratory arrest two months after first being injected with paliperidone palmitate (150 mg). The patient was intubated for two days; on the third day after extubation, he became delirious and was given haloperidol (10 mg IM). During the following hour, he developed an upper airway obstruction and was reintubated. His clinical condition was improved by intravenous methylprednisolone. There was also a case of oral paliperidone-induced angioedema. A 19-year-old female with schizophrenia from Turkey (Table 1) presented with facial edema two hours after her oral paliperidone dosage had been increased from 3 to 6 mg. The edema resolved within one week of the oral paliperidone dosage being reverted to 3 mg, but it re-emerged following rechallenge with 6 mg oral paliperidone[11].
| Paliperidone form | Age/sex, country | Year | Underlying disease | Dose | Onset | Co-medication | Medication allergy | Presentations | Duration | Naranjo score[13] | Conclusion |
| Oral | 19/F, Turkey[11] | 2015 | Schizophrenia | 6 mg/d | 2 h | None | None | Swelling of face, lips, & eyelids | 1 wk | 6 | Facial angioedema |
| Injectable | 30/M, Greece[12] | 2017 | Schizophrenia | 150 mg monthly | 2nd month | None | Breathing difficulty from risperidone + lamotrigine | Sudden suffocation; seizure; & collapse in the house. | 2 d | 5 | Acute laryngeal edema with respiratory arrest |
| Injectable | 79/F, Thailand | This report | Major neurocognitive disorder due to Alzheimer’s disease, type 2 diabetes, dyslipidemia, hypertension | Induction doses (150 mg; 100 mg) | 17th day | Donepezil, metformin, simvastatin, felodipine, atenolol | Aspirin | Intermittent swelling of face, lips, and eyelids | 23 d | 6 | Facial angioedema |
This report presents a rare case of paliperidone palmitate-induced angioedema. Paliperidone palmitate was administered to a 79-year-old elderly patient who had a major neurocognitive disorder due to Alzheimer’s disease with behavioral disturbances and psychotic symptoms. She developed intermittent facial edema 17 d after the first administration, and the symptoms lasted for another 23 d (to day 40 after the initial paliperidone palmitate dose). This might be explained by the long half-life of paliperidone palmitate, which ranges from 24 to 49 d[15].The duration of the symptoms of medication-induced allergic angioedema may vary among the few reported cases due to the unclear pathophysiological pathways of the drug and the possible influence of culprit medications. Although this patient received antihypertensive medications (such as calcium channel antagonists, which could induce angioedema), the duration of the oral antihypertensive administration was not compatible with the onset of the symptoms[19]. Of interest, the angioedema in this case report exhibited only with the paliperidone palmitate injection, not with the oral paliperidone. This phenomenon has also been noted in case reports of paliperidone[12] and risperidone[20] usage. The mechanism remains unclear. We hypothesized that increasing dose might be a key factor in developing angioedema in this patient because angioedema did not occur when oral paliperidone 6 mg (equivalent to paliperidone palmitate injection 75 mg)[14] or risperidone 2 mg (equivalent to paliperidone palmitate injection 50 mg)[21] were given, but developed at higher dose of paliperidone palmitate injection 150 mg and 100 mg.
Both immunological and non-immunological reactions could be suspected in this case. Although the complement level and serum tryptase were not investigated in this patient, a non-immunological reaction was more likely due to the delayed onset of angioedema and the absence of an urticarial rash. Paliperidone is the active metabolite of risperidone, and the mechanism of angioedema from paliperidone may resemble that of risperidone. Risperidone can suppress the activity of the C1-inhibitor, thereby increasing anaphylatoxin level, which in turn increases vasodilation and vascular permeability. Moreover, risperidone can also induce bradykinin aggregation and stimulate nitric oxide and prostacyclin, all of which increase vascular permeability[18]. Should angioedema from paliperidone be caused by an immunological reaction, cross-reactivity with other antipsychotics (clozapine-olanzapine[17], clozapine-olanzapine-quetiapine[9], and haloperidol-iloperidone[7]) and paliperidone could occur. A decision to change to alternative drugs warrants careful consideration during treatment.
Discontinuation of the paliperidone palmitate proved to be adequate for the management of angioedema in this case. In concordance with previous reported cases, dose reduction[11,22] or discontinuation (with[9] or without[8,17] an antihistamine and corticosteroid) of the culprit antipsychotic is sufficient for the management of antipsychotic-induced angioedema. However, in more severe cases in which the respiratory tract could be involved, the administration of an additional corticosteroid, antihistamine, and/or epinephrine was warranted[7,20].
The present case report has some mentionable strengths. Firstly, unlike earlier reports, our pictures clearly show the presence of angioedema. Moreover, as the patient’s daughters, both with master’s degrees, were circumspect, the information obtained was highly accurate. A limitation of this case report was the lack of immunology testing; it is therefore difficult to provide the exact mechanism of angioedema.
Paliperidone palmitate-induced angioedema can present with intermittent facial edema which may be overlooked. The symptoms remit with medication discontinuation. The symptoms of angioedema in this case were relatively mild relative to those of the previously reported case[12], and the patient’s condition was not complicated by life-threatening anaphylaxis. However, there has been a report that the degree of symptoms of angioedema arising from paliperidone may be dose-dependent[11]. Therefore, when introducing paliperidone or increasing its dosage, patients should be closely monitored for adverse reactions, especially psychiatric patients who are unable to detect their illness.
The authors gratefully acknowledge the patient’s daughters, who provided useful information regarding their beloved mother.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Royal College of Psychiatrists of Thailand.
Specialty type: Psychiatry
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Flyckt L, Hosak L S-Editor: Zhang H L-Editor: A P-Editor: Li JH
| 1. | Gilday E, Nasrallah HA. Clinical pharmacology of paliperidone palmitate a parenteral long-acting formulation for the treatment of schizophrenia. Rev Recent Clin Trials. 2012;7:2-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Chue P, Chue J. A review of paliperidone palmitate. Expert Rev Neurother. 2012;12:1383-1397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Jang S, Woo J. Five Month-Persistent Extrapyramidal Symptoms following a Single Injection of Paliperidone Palmitate: A Case Report. Clin Psychopharmacol Neurosci. 2017;15:288-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Gopal S, Berwaerts J, Nuamah I, Akhras K, Coppola D, Daly E, Hough D, Palumbo J. Number needed to treat and number needed to harm with paliperidone palmitate relative to long-acting haloperidol, bromperidol, and fluphenazine decanoate for treatment of patients with schizophrenia. Neuropsychiatr Dis Treat. 2011;7:93-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Inomata N. Recent advances in drug-induced angioedema. Allergol Int. 2012;61:545-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | HINE FR. Severe angioneurotic edema during chlorpromazine therapy. Am J Psychiatry. 1958;114:942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Muzyk AJ, Cvelich RG, Kincaid BR, Preud'homme XA. Angioedema occurring in patient prescribed iloperidone and haloperidol: a cross-sensitivity reaction to antipsychotics from different chemical classes. J Neuropsychiatry Clin Neurosci. 2012;24:E40-E41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Soumya RN, Grover S, Dutt A, Gaur N. Angioneurotic edema with risperidone: a case report and review of literature. Gen Hosp Psychiatry. 2010;32:646.e1-646.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Williams GD. Cross-reaction of angioedema with clozapine, olanzapine, and quetiapine: A case report. Ment Health Clin. 2019;9:315-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Mohan T, Bastiampillai T, Dhillon R. Ziprasidone-induced angioedema: a case report. J Clin Psychiatry. 2009;70:1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Yucel A, Yucel N, Ozcan H, Saritemur M. Dose-Dependent Paliperidone Associated With Angioedema. J Clin Psychopharmacol. 2015;35:615-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Papadopoulou A, Gkikas K, Efstathiou V, Gkikas I, Kokoris S, Lagari V, Papageorgiou C, Douzenis A, Siafakas N, Rizos EN. Angioedema Associated With Long-Acting Injectable Paliperidone Palmitate: A Case Report. J Clin Psychopharmacol. 2017;37:730-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7061] [Cited by in F6Publishing: 7670] [Article Influence: 178.4] [Reference Citation Analysis (0)] |
| 14. | Gopal S, Gassmann-Mayer C, Palumbo J, Samtani MN, Shiwach R, Alphs L. Practical guidance for dosing and switching paliperidone palmitate treatment in patients with schizophrenia. Curr Med Res Opin. 2010;26:377-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Morris MT, Tarpada SP. Long-Acting Injectable Paliperidone Palmitate: A Review of Efficacy and Safety. Psychopharmacol Bull. 2017;47:42-52. [PubMed] [Cited in This Article: ] |
| 16. | Jarema M, Bieńkowski P, Heitzman J, Parnowski T, Rybakowski J. Paliperidone palmitate: effectiveness, safety, and the use for treatment of schizophrenia. Psychiatr Pol. 2017;51:7-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Tatar ZB, Oflaz S, Baran B. A case of late-onset angioedema associated with clozapine and redevelopment of angioedema with olanzapine. J Clin Psychopharmacol. 2014;34:523-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Kalambay J, Ghazanfar H, Martes Pena KA, Munshi RA, Zhang G, Patel JY. Pathogenesis of Drug Induced Non-Allergic Angioedema: A Review of Unusual Etiologies. Cureus. 2017;9:e1598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Agostoni A, Cicardi M. Drug-induced angioedema without urticaria. Drug Saf. 2001;24:599-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Güneş F, Batgi H, Akbal A, Canatan T. Angioedema - an unusual serious side effect of risperidone injection. Clin Toxicol (Phila). 2013;51:122-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Russu A, Kern Sliwa J, Ravenstijn P, Singh A, Mathews M, Kim E, Gopal S. Maintenance dose conversion between oral risperidone and paliperidone palmitate 1 month: Practical guidance based on pharmacokinetic simulations. Int J Clin Pract. 2018;72:e13089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Cooney C, Nagy A. Angio-oedema associated with risperidone. BMJ. 1995;311:1204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |