Published online Sep 6, 2018. doi: 10.12998/wjcc.v6.i9.301
Peer-review started: April 2, 2018
First decision: May 24, 2018
Revised: June 1, 2018
Accepted: June 26, 2018
Article in press: June 27, 2018
Published online: September 6, 2018
The clivus is an atypical metastatic site for renal clear cell carcinoma (RCCC). Here we report a 54 year old man with acute cavernous sinus syndrome. Brain magnetic resonance imaging identified a clival-based lesion with associated bony erosion. The patient underwent endoscopic endonasal biopsy and partial resection of the clival mass. Because histologic examination of the resected specimen resulted in a diagnosis of RCCC, contrast-enhanced computed tomography scan of the abdomen was performed and showed an enhanced left renal mass. The patient subsequently underwent laparoscopic left radical nephrectomy and gamma knife was planned for the residual clival lesion. We also retrospectively reviewed available published reports on clival metastases, specifically those from RCCC, since 1990.
Core tip: Clival metastasis is an extremely rare presentation of renal clear cell carcinoma. The symptom of sudden onset of cranial neuropathy, most commonly involving the abducens nerve, and findings on radiologic examination are crucial for making an early diagnosis. Histopathological diagnosis and resection of the clival mass can be safely achieved through an endoscopic endonasal approach. Multidisciplinary management, including surgery, stereotactic radiotherapy and tumor-targeted agents, is often required to prolong survival and maximize the quality of life for patients with metastatic renal cell carcinoma.
- Citation: Zhang WQ, Bao Y, Qiu B, Wang Y, Li ZP, Wang YB. Clival metastasis of renal clear cell carcinoma: Case report and literature review. World J Clin Cases 2018; 6(9): 301-307
- URL: https://www.wjgnet.com/2307-8960/full/v6/i9/301.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i9.301
Renal cell carcinoma (RCC) is the ninth most common cancer in men, and the fourteenth most common in women[1]. Approximately 90% of kidney cancers are RCCs[2,3]. The most common subtype is renal clear cell carcinoma (RCCC), comprising about 75%-80% of RCCs in surgical series[4]. Almost one-third of patients with RCC present with metastatic disease. The usual metastatic sites are lungs (45.2%), bone (29.5%), lymph nodes (21.8%), liver (20.3%), adrenal gland (8.9%), and brain (8.1%)[5]. Tumors originating primarily in the clivus region are very rare. Chordomas, which are the most frequent tumor affecting that region, represent only 0.15% of all intracranial tumors and 6% of all skull base tumors[6,7]. Common differential diagnoses of clival neoplasms are meningioma, chordoma, lymphoma, pituitary adenoma, nasopharyngeal carcinoma, bone marrow reconversion, and metastatic lesions[8]. Clival metastases from RCCC account for a small proportion of clival tumors. Few cases have been reported.
A 54-year-old man presented to the ophthalmology outpatient clinic of our institution with asthenia for one month and acute onset drooping of his right eyelid with diplopia for fourteen days. He had smoked ten cigarettes daily for 30 years. On admission, physical examination revealed palsies of right cranial nerves III, IV, VI, and V2 manifesting as right-sided ptosis, diplopia, and decreased sensation over the right cheek. Both pupils were normal in size and reacted to light.
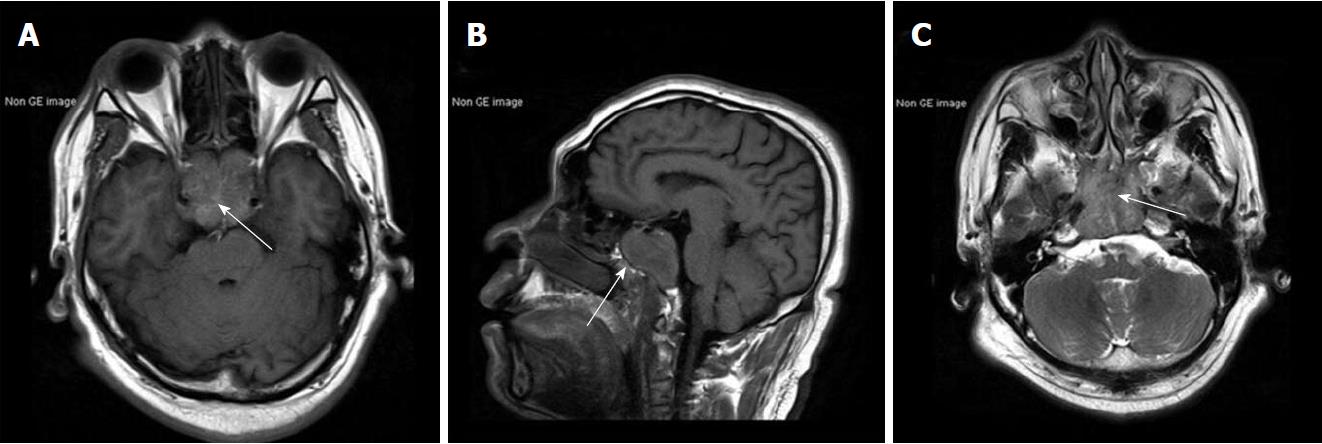
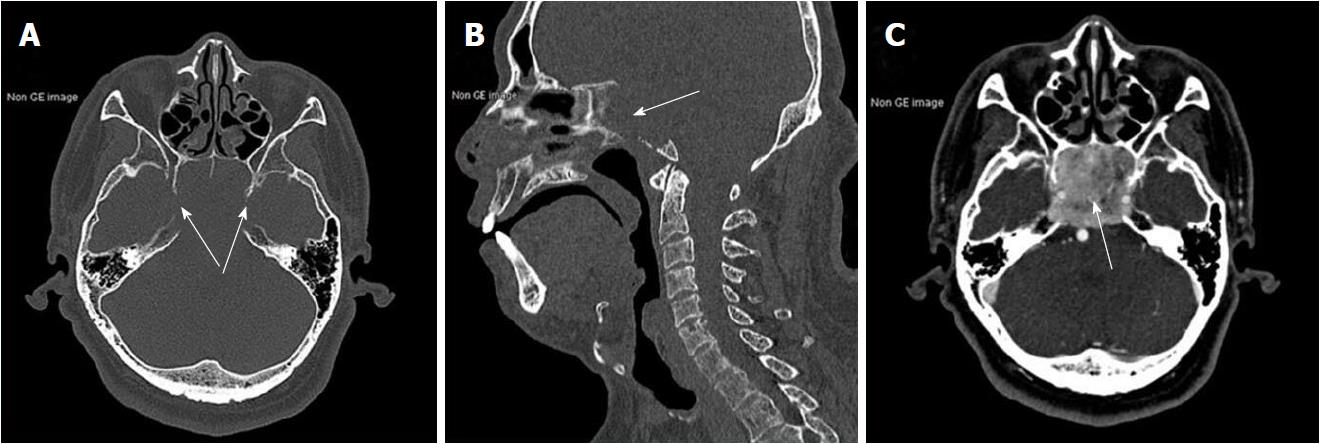
Results of routine laboratory tests were normal apart from a high urinary red blood cell (RBC) of 14.80/hpf. A non-contrast brain magnetic resonance imaging (MRI) showed a mass with irregular margins in the clivus. The mass was invading the sphenoid and cavernous sinuses bilaterally and encasing both carotid arteries. It was isointense on T1-weighted images and slightly hyperintense on T2-weighted images (Figure 1). Brain computed tomography (CT) scan showed obvious osteolysis of the cranial base involving the clivus and both petrous apexes. There was marked enhancement of the clival lesion following intravenous contrast injection (Figure 2).
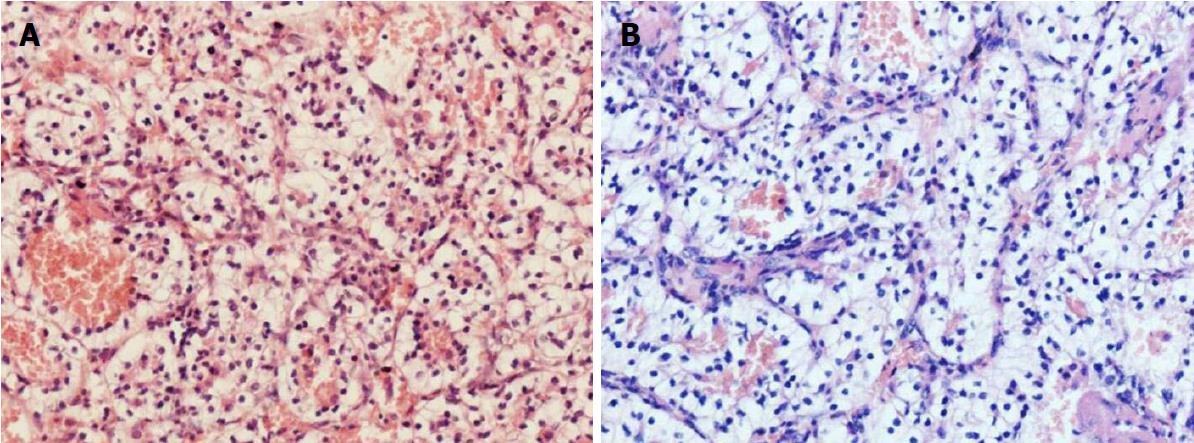
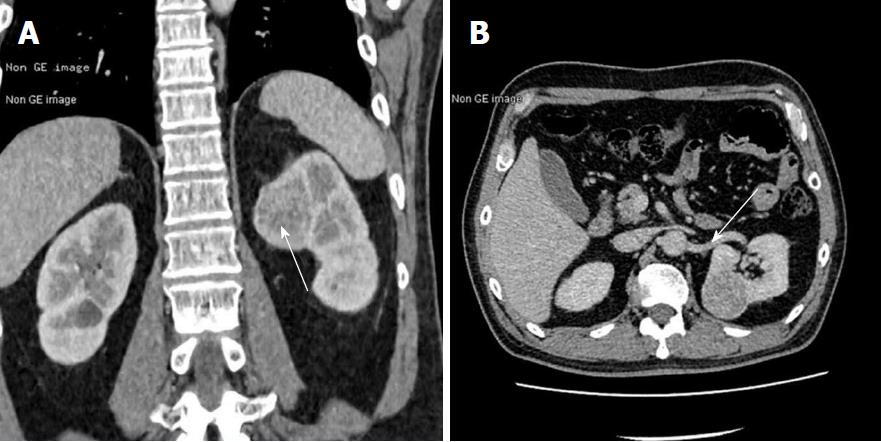
To obtain a tissue diagnosis and decompress the cavernous sinus, the patient underwent endoscopic endonasal transclival resection of the lesion. Intraoperatively, the tumor was noted to be firm and highly hemorrhagic. Massive bleeding necessitated interruption of the procedure and caused a marked drop in blood pressure. Hemostasis was achieved once the tumor had been partially resected and 400 mL of blood transfused. An early postoperative CT scan showed no evidence of major complications. Immediately after the procedure, the patient’s right-sided ptosis and diplopia improved slightly. Pathological examination of the operative specimen resulted in a diagnosis of clear cell carcinoma (Figure 3A). Ki67 was expressed in 10%-20% of the neoplastic cells and immunohistochemical staining was positive for cytokeratin, vimentin, CK8/18, paired box gene 8 and cluster of differentiation 10. On the sixth postoperative day, the patient underwent a contrast-enhanced CT scan of the abdomen, which showed a slightly inhomogeneous, enhancing, roundish mass in the upper pole of the left kidney. No tumor thrombus in the left renal vein or retroperitoneal lymphadenopathy was detected. Contrast-enhanced thoracic CT scan showed no pulmonary metastases (Figure 4). Laparoscopic left radical nephrectomy was performed twelve days after the clival surgery. A 4 cm × 4 cm × 4.5 cm tumor and a 2 cm × 2.5 cm × 2.5 cm isolated tumor satellite focus were noted in the posterosuperior part of the left kidney. The patient did not develop any postoperative complications such as abdominal infection and was discharged ten days after the second surgery. Histopathologic examination of the resected kidney resulted in a diagnosis of RCCC consistent with that of the intracranial tumor (Figure 3). The patient underwent gamma knife for the residual clival lesion one month later. An MRI performed one month after this procedure demonstrated expected postoperative changes. At the four month follow-up, the patient was in good general condition, though his right cavernous sinus syndrome had not improved. Further follow-up is ongoing.
Most reports of metastases to the clivus have been in the form of case reports, case images, or small series[9]. We performed an extensive review of available reports on clival metastases from RCCC and identified nine patients, including the present case (Table 1). The male/female ratio was 5:4 and mean age was 54 years (range 27-62 years). The clival metastasis was diagnosed first in seven of the nine patients. The main clinical manifestations were sixth nerve palsy, headache, and diplopia. Our results are similar to those reported by Dekker et al[10]. All reported primary RCCCs were unilateral.
| Ref. | Age/sex | Symptoms | First diagnosis and interval | Position and size of Primary Tumor | Surgery for clival metastasis | Surgery for primary tumor | Additional treatment | Follow-up |
| Fumino et al[28] | 58/M | Diplopia | Clival metastasis, NA | The left kidney, 6 cm × 4 cm | None | Left radical nephrectomy | Radiotherapy to the clivus | NA |
| Endo et al[29] | 59/M | Occipital pain, dysarthria, CN XII palsy | Clival metastasis, NA | The right kidney, 9 cm | None | None | Radiotherapy for the clivus | DOD (6 mo) |
| Sepúlveda et al[30] | 62/M | Sixth nerve palsy, dysarthria, right tongue deviation and right facial paralysis | Clival metastasis, NA | The right kidney, NA | Biopsy | None | Radiotherapy and palliative care | NA |
| Patel et al[31] | 59/F | Headaches and acute onset cranial nerve neuropathies | Clival metastasis, NA | The left kidney, NA | Endoscopic endonasal near complete resection | None | Palliative radiation treatment | NA |
| Mendelson et al[32] | 59/F | Headaches and dropping of left eye with double vision | Clival metastasis, NA | The left kidney, NA | Endoscopic decompression of clival lesion | None | Palliative radiotherapy for renal mass | NA |
| Mani et al[12] | 55/M | Headache and diplopia photophobia of right eye | Clival metastasis, NA | The left kidney, 6.5 cm × 6.0 cm × 5.5 cm | None | Biopsy | External beam radiation therapy and supportive palliative care | DOD (6 mo) |
| Gil Salu et al[33] | 56/F | Diplopia | Primary RCCC, 8 yr | The right kidney, NA | Endoscopic endonasal partial resection | Right nephrectomy | NA | NA |
| Santhosh et al[34] | 27/F | Pain in right lower limb | Primary RCCC, NA | The right kidney, NA | None | Right radical nephrectomy | Immunotherapy | AWD (28 mo) |
| Zhang et al | 54/M | Asthenia and drooping of his right eyelid with diplopia | Clival metastasis, 6 d | The left kidney, 4 cm × 4 cm × 4.5 cm | Endoscopic endonasal biopsy and partial resection | Laparoscopic left radical nephrectomy | Gamma knife for residual clival lesion | AWD (4 mo) |
The clivus, part of the skull base, is located between the foramen magnum and dorsum sellae and lies deep in the midline in intimate relationship with various critical neurovascular structures[9,11]. In particular, the sixth nerve is very prone to involvement by tumors growing from the clivus and petroclival regions because of its long serpentine course from the brainstem to the superior orbital fissure[12]. Abducens nerve palsy was the presenting manifestation in 46% of patients with chordoma and 47% of those with chondrosarcoma reported by Deconde et al[13]. Above 40% of clival metastases present with isolated sixth nerve palsy according to the review conducted by Dekker et al[10]. That our patient’s tumor extended into the right cavernous sinus may explain the presence of multiple cranial nerve palsies. The possibility of metastatic RCCC should be considered in patients with a clival lesion and cranial neuropathies. Patients with RCC usually present with the classical triad of macroscopic hematuria, abdominal mass, and flank pain. However, many such tumors are asymptomatic and detected incidentally on health check-ups or imaging examinations[2].
Most reported diagnoses of clival metastases are based on imaging examinations including CT, MRI, positron emission tomography scan with CT (PET-CT) and radionuclide bone scans using technetium or gallium[14]. The superior sensitivity of MRI in detecting bone marrow diseases allows precise localization and evaluation of the signal characteristics of clival lesions[8]. Because of its central location, the clivus is best seen on a midsagittal view on MRI. A normal clivus characteristically shows mild enhancement. Low-intensity clival lesions in the marrow tend to be isointense with normal marrow on contrast enhancement. Thus, unenhanced images are more sensitive for detecting clival lesions[8,15]. The usual normal adult clival signal is iso- or hyperintense compared with the pons on T1-weighted images and approximately isointense with the pons on T2-weighted images. Replacement of fatty bone marrow by clival lesions may explain why most clival lesions appear hypointense on T1-weighted images and hyperintense on T2-weighted images[8,12]. Integrated PET-CT has been shown to be an effective means of preoperative staging and follow-up surveillance of patients with skull base tumors (overall sensitivity of 77% and specificity of 81%)[14]. However, clival metastases do not appear to have any distinctive radiological features. Radiologic examinations alone have limited ability to distinguish metastases from primary lesions such as chordomas and chondrosarcomas[16].
An endoscopic endonasal approach is reported as a promising option for managing clival lesions with minimal morbidity[17]. With the use of a rigid endoscope and angled telescopes, an endoscopic endonasal approach provides a significantly wider and better-illuminated surgical field than traditional open surgeries[18]. In the articles we reviewed, an endoscopic endonasal approach was used in four patients to obtain a biopsy or achieve partial resection. Endoscopic skull base surgeries aided by surgical navigation systems based on preoperative imaging, such as CT and MRI, allow the surgeon to recognize critical structures and maximize the precision of the resection[18]. The application of intraoperative navigated angiosonography techniques for skull base surgery may be helpful in approaching the tumor and avoiding vascular damage[19]. However, endoscopic endonasal approaches have some limitations, including limited working space, reduced maneuverability, and the need for special instrumentation. It is important to carefully select the most appropriate approach for each patient on the basis of patient- and tumor-related factors as well as the experience of the surgeon.
RCC can be associated with a favorable outcome when diagnosed at an early stage[20]. Unfortunately, 30% of these patients have metastatic disease at diagnosis or after treatment of local disease[20]. The largest published retrospective study comprised 286 patients with brain metastases from RCC and their median survival was 9.63 mo[21]. Disease-specific prognostic factors include Karnofsky performance status and the number of brain metastases. A study by Vickers et al[22] revealed that histologic diagnoses of RCCC are more common in patients with brain metastases than in those with metastases to other sites (96% of these patients had clear cell histology). The median survival of patients with brain metastases from RCC is reportedly 14.4 mo, whereas that of patients with RCC without brain metastases is 19.0 mo[22].
Although multiple advances have been made in systemic therapy for RCC, management of metastatic RCC (mRCC) is still challenging[4]. Surgical treatment of our patient, namely partial removal of the metastatic lesion, nephrectomy, and gamma knife treatment were performed successively because he had a solitary metastasis and his physical status was good. The role of radiotherapy in the treatment of RCC remains unclear and it is not recommended[23]. However, several case series have reported that stereotactic radiotherapy is a valuable means of reducing local symptoms from tumor bulk and stabilizing the growth of metastatic lesions in both cranial and extracranial sites[2,24]. Approximately 70% of RCCCs are associated with loss of function of the von Hippel-Lindau gene (VHL). VHL can result in high concentrations of hypoxia inducible factor (HIF) and vascular endothelial growth factor (VEGF), which facilitates tumor-associated angiogenesis[25]. Tumor-targeted therapies for RCCC can combat tumor angiogenesis and inhibit tumor cell proliferation. Extended clinical trials of sunitinib and sorafenib have shown that these targeted drugs improve the efficacy of treatment of brain metastasis from RCCC[26,27]. A combination of surgery, stereotactic radiotherapy, and non-targeted and/or targeted agents is often required for management of mRCC. Clinicians should always focus on the goals of treatment and tailor an individual treatment plan to ensure the best possible outcomes.
A 54-year-old man with a history of smoking presented with asthenia and acute right cavernous sinus syndrome.
Palsies of right cranial nerves III, IV, VI, and V2, likely caused by intracranial lesions.
Differential diagnoses included clival chondroma, clival chondrosarcoma, intraosseous lymphoma, and meningioma.
Results of routine laboratory tests were normal, apart from a high urinary red blood cell of 14.80/hpf.
Non-contrast brain magnetic resonance imaging demonstrated a mass lesion in the clivus with irregular margins that had invaded the sphenoid and cavernous sinus bilaterally. Brain computed tomography (CT) scan showed obvious osteolysis of the cranial base involving the clivus and both petrous apexes. Contrast-enhanced CT scan of the abdomen showed a slightly inhomogeneous, enhancing, roundish mass in the upper pole of the left kidney.
Histopathological examination of the operative specimens revealed clear cells in an alveolar pattern, being separated by a reticular meshwork of thin walled vessels.
Endoscopic endonasal partial resection of the clival metastasis. Laparoscopic left radical nephrectomy of the primary renal clear cell carcinoma (RCCC). Gamma knife for the residual clival lesion.
Few reports of clival metastasis from RCCC have been published. These tumors tend to be very aggressive, as evidenced by presentation at an advanced stage of the disease. Multidisciplinary management is necessary.
The Karnofsky Performance Scale is a means of classifying patients’ functional impairment. Scores can be used to compare effectiveness of different therapies and assess the prognosis of individual patients. The lower the Karnofsky score, the worse the prognosis.
Clival metastasis from RCCC should be considered in the differential diagnosis of bony lesions of the clivus in patients with cranial neuropathy of sudden onset. Early diagnosis, clinical experience, and multidisciplinary management are crucial for effective treatment of such lesions.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Budai B, Vidal EIO S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW
| 1. | Medina-Rico M, Ramos HL, Lobo M, Romo J, Prada JG. Epidemiology of renal cancer in developing countries: Review of the literature. Can Urol Assoc J. 2018;12:E154-E162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Graves A, Hessamodini H, Wong G, Lim WH. Metastatic renal cell carcinoma: update on epidemiology, genetics, and therapeutic modalities. Immunotargets Ther. 2013;2:73-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Nguyen MM, Gill IS, Ellison LM. The evolving presentation of renal carcinoma in the United States: trends from the Surveillance, Epidemiology, and End Results program. J Urol. 2006;176:2397-2400; discussion 2400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 240] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 4. | Gong J, Maia MC, Dizman N, Govindarajan A, Pal SK. Metastasis in renal cell carcinoma: Biology and implications for therapy. Asian J Urol. 2016;3:286-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Bianchi M, Sun M, Jeldres C, Shariat SF, Trinh QD, Briganti A, Tian Z, Schmitges J, Graefen M, Perrotte P. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 429] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 6. | Korten AG, ter Berg HJ, Spincemaille GH, van der Laan RT, Van de Wel AM. Intracranial chondrosarcoma: review of the literature and report of 15 cases. J Neurol Neurosurg Psychiatry. 1998;65:88-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Samii A, Gerganov VM, Herold C, Hayashi N, Naka T, Mirzayan MJ, Ostertag H, Samii M. Chordomas of the skull base: surgical management and outcome. J Neurosurg. 2007;107:319-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Kimura F, Kim KS, Friedman H, Russell EJ, Breit R. MR imaging of the normal and abnormal clivus. AJR Am J Roentgenol. 1990;155:1285-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Okudo J, Anusim N. Unusual Spread of Renal Cell Carcinoma to the Clivus with Cranial Nerve Deficit. Case Rep Neurol Med. 2016;2016:9184501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Dekker SE, Wasman J, Yoo KK, Alonso F, Tarr RW, Bambakidis NC, Rodriguez K. Clival Metastasis of a Duodenal Adenocarcinoma: A Case Report and Literature Review. World Neurosurg. 2017;100:62-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Mohyeldin A, Prevedello DM, Jamshidi AO, Ditzel Filho LF, Carrau RL. Nuances in the treatment of malignant tumors of the clival and petroclival region. Int Arch Otorhinolaryngol. 2014;18:S157-S172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Mani A, Yadav P, Paliwal VK, Lal H. Isolated clival metastasis: a rare presentation of renal cell carcinoma. BMJ Case Rep. 2017;2017:pii: bcr-2017-221570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Deconde AS, Sanaiha Y, Suh JD, Bhuta S, Bergsneider M, Wang MB. Metastatic disease to the clivus mimicking clival chordomas. J Neurol Surg B Skull Base. 2013;74:292-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Gil Z, Even-Sapir E, Margalit N, Fliss DM. Integrated PET/CT system for staging and surveillance of skull base tumors. Head Neck. 2007;29:537-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Chaljub G, Van Fleet R, Guinto FC Jr, Crow WN, Martinez L, Kumar R. MR imaging of clival and paraclival lesions. AJR Am J Roentgenol. 1992;159:1069-1074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Movsas TZ, Balcer LJ, Eggenberger ER, Hess JL, Galetta SL. Sixth nerve palsy as a presenting sign of intracranial plasmacytoma and multiple myeloma. J Neuroophthalmol. 2000;20:242-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 17. | Carrabba G, Dehdashti AR, Gentili F. Surgery for clival lesions: open resection versus the expanded endoscopic endonasal approach. Neurosurg Focus. 2008;25:E7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Solares CA, Fakhri S, Batra PS, Lee J, Lanza DC. Transnasal endoscopic resection of lesions of the clivus: a preliminary report. Laryngoscope. 2005;115:1917-1922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Prada F, Del Bene M, Casali C, Saladino A, Legnani FG, Perin A, Moiraghi A, Richetta C, Rampini A, Mattei L. Intraoperative Navigated Angiosonography for Skull Base Tumor Surgery. World Neurosurg. 2015;84:1699-1707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1329] [Cited by in F6Publishing: 1264] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 21. | Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 689] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 22. | Vickers MM, Al-Harbi H, Choueiri TK, Kollmannsberger C, North S, MacKenzie M, Knox JJ, Rini BI, Heng DY. Prognostic factors of survival for patients with metastatic renal cell carcinoma with brain metastases treated with targeted therapy: results from the international metastatic renal cell carcinoma database consortium. Clin Genitourin Cancer. 2013;11:311-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Kenney PA, Wood CG. Integration of surgery and systemic therapy for renal cell carcinoma. Urol Clin North Am. 2012;39:211-231, vii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Lwu S, Goetz P, Monsalves E, Aryaee M, Ebinu J, Laperriere N, Menard C, Chung C, Millar BA, Kulkarni AV. Stereotactic radiosurgery for the treatment of melanoma and renal cell carcinoma brain metastases. Oncol Rep. 2013;29:407-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991-5004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 699] [Cited by in F6Publishing: 682] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 26. | Guo J, Ma J, Sun Y, Qin S, Ye D, Zhou F, He Z, Sheng X, Bi F, Cao D. Chinese guidelines on the management of renal cell carcinoma (2015 edition). Ann Transl Med. 2015;3:279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 27. | Stukalin I, Alimohamed N, Heng DY. Contemporary Treatment of Metastatic Renal Cell Carcinoma. Oncol Rev. 2016;10:295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Fumino M, Matsuura H, Hayashi N, Arima K, Yanagawa M, Kawamura J. [A case of renal cell carcinoma with metastasis in clivus presenting as diplopia]. Hinyokika Kiyo. 1998;44:319-321. [PubMed] [Cited in This Article: ] |
| 29. | Endo K, Okano R, Kuroda Y, Yamada S, Tabei K. Renal cell carcinoma with skull base metastasis preceded by paraneoplastic signs in a chronic hemodialysis patient. Intern Med. 2001;40:924-930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Sepúlveda I, Platin E, Klaassen R, Spencer ML, García C, Alarcón R, Ulloa D. Skull base clear cell carcinoma, metastasis of renal primary tumor: a case report and literature review. Case Rep Oncol. 2013;6:416-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Patel AA, Kuperan AB, Patel CR, Sharer LR, Liu JK, Eloy JA. Renal cell carcinoma with metastasis to the clivus. NINJ. 2013;. [Cited in This Article: ] |
| 32. | Mendelson ZS, Patel AA, Eloy JA, Liu JK. Endoscopic palliative decompression of the cavernous sinus in a rare case of a metastatic renal cell carcinoma to the clivus. Br J Neurosurg. 2015;29:430-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Gil Salu JL. Metástasis clival tardía de carcinoma renal de células claras. Neurocirugia. 2017;28:296. [Cited in This Article: ] |
| 34. | Santhosh KD. Immunotherapy to the rescue in advanced RCC. Available from: http://oncologypro.esmo.org/content/download/125614/2375060/file/2017-ESMO-Preceptorship-I-O-Participant-Clinical-Case-Discussion-Immunotherapy-Advanced-RCC-Santhosh-Kumar-Devadas.pdf. [Cited in This Article: ] |