Introduction
Status epilepticus (SE) is a common neurological emergency with an incidence of 36.1 per 100,000 adults per year. Reference Leitinger, Trinka and Giovannini1 Cerebrospinal fluid (CSF) analysis is routinely used to determine the potential causes. Pleocytosis of CSF has been reported in up to 34% of adult patients who have focal seizures, generalized seizures, or SE Reference Prokesch, Rimland, Petrini and Fein2-Reference Aminoff and Simon4 regardless of the cause. However, some of the more recent studies that have focused on SE demonstrate the prevalence of CSF pleocytosis after SE to be around 6% without exclusion of all secondary causes and consideration of the effect of time. Reference Malter, Choi and Fink5,Reference Tumani, Jobs, Brettschneider, Hoppner, Kerling and Fauser6
It is well known that secondary causes of seizures may lead to CSF pleocytosis, including infection, inflammation, ischemic and inflammatory vascular pathology, neoplastic, neurodegenerative diseases, and iatrogenic disturbances. Reference Egelund, Ertner, Kristensen, Jensen, Benfield and Brandt7 Since management of SE varies greatly depending on the cause, it is quite important to know whether increased cells are due to SE per se, or from the underlying cause. Reference Egelund, Ertner, Kristensen, Jensen, Benfield and Brandt7 However, there is a lack of consensus whether SE itself results in CSF pleocytosis, especially in the first 24 hours of onset. Reference Prokesch, Rimland, Petrini and Fein2-Reference Tumani, Jobs, Brettschneider, Hoppner, Kerling and Fauser6,Reference Schmidley and Simon8
In the absence of an underlying infectious or inflammatory etiology, a proposed mechanism for CSF pleocytosis in prolonged seizures is developing neuroinflammation as a result of excitotoxicity process of SE. Reference Vezzani, French, Bartfai and Baram9-Reference Xu, Long, Tang, Zhang, Hut and Tang14 Affected glial cells, neurons, and endothelial cells, release a host of inflammatory mediators including interleukins, interferons, tumor necrosis factors, and growth factors. The released cytokines lead to a number of downstream effects such as increased permeability of blood brain barrier (BBB) Reference Bolton and Perry15 and increased production of second-line chemokines such as monocyte chemoattractant protein-1 and macrophage inflammatory protein 1-alpha by glial cells and neurons. Reference Kalehua, Nagel and Whelchel16,Reference Fabene, Bramanti and Constantin17 These chemokines guide leukocyte migration through the BBB and into the area of injury. Reference Vezzani, French, Bartfai and Baram9,Reference Fabene, Bramanti and Constantin17 Leukocyte recruitment to the site of injury has been demonstrated to raise within 18 hours and peak around 24 hours after the excitotoxicity related to prolonged seizures in rats. Reference Dinkel, MacPherson and Sapolsky18 This timeline of cytotoxic-induced inflammation in animal models agrees with the CSF pleocytosis peak noted a day following seizures in clinical studies. Reference Aminoff and Simon4,Reference Schmidley and Simon8
Based on the above, we hypothesized that CSF pleocytosis seen within the first 24 hours following SE is most likely due to the underlying etiology as opposed to the prolonged seizure itself. The main objective of our study was to determine whether SE, without infectious or inflammatory cause, leads to pleocytosis within 24 hours and whether there is a time effect for this phenomenon. Our secondary objective was to see if there are differences in CSF findings, serum white blood cell (WBC) count, clinical characteristics, and outcomes between patients with and without CSF pleocytosis.
Additionally, there have been studies suggesting that seizures themselves can cause elevated lactate content, BBB disturbances, and elevated protein in the CSF. Reference Malter, Choi and Fink5,Reference Tumani, Jobs, Brettschneider, Hoppner, Kerling and Fauser6,Reference Calabrese, Gruemer, James, Hranowsky and DeLorenzo19 However, this has never been tested in a refractory SE population. Therefore, our tertiary objective was to assess if these abnormalities occur in refractory SE and whether there are any relationships between clinical SE characteristics and CSF parameters or clinical outcomes.
Methods
Study Design
We performed a historical cohort study of adult patients (age ≥ 18 years) admitted with SE to the intensive care unit (ICU) of Vancouver General Hospital between March 2010 and May 2019. Our study was approved by the University of British Columbia Research Ethics Board. Patients were identified using the ICU database and with the following admission diagnostic codes: APACHE II (SE or uncontrolled seizures) or APACHE IV (seizures, primary-no structural brain disease). SE was defined according to the 2015 definition by ILAE Task Force. Reference Trinka, Cock and Hesdorffer20
Inclusion and Exclusion Criteria
Patients were included if they underwent a lumbar puncture (LP) for CSF analysis within 5 days of onset of SE. Patients were excluded if either SE was not confirmed after chart review, or there was a traumatic tap (RBC more than 1000 × 106/L), or CSF was obtained from an external ventricular drain, or there was subarachnoid hemorrhage or if the patient’s electronic chart did not contain CSF WBC count needed for our primary objective (Figure 1). No formal sample size calculation was performed. The sample size was one of convenience and represents the number of patients available for analysis in the time period.
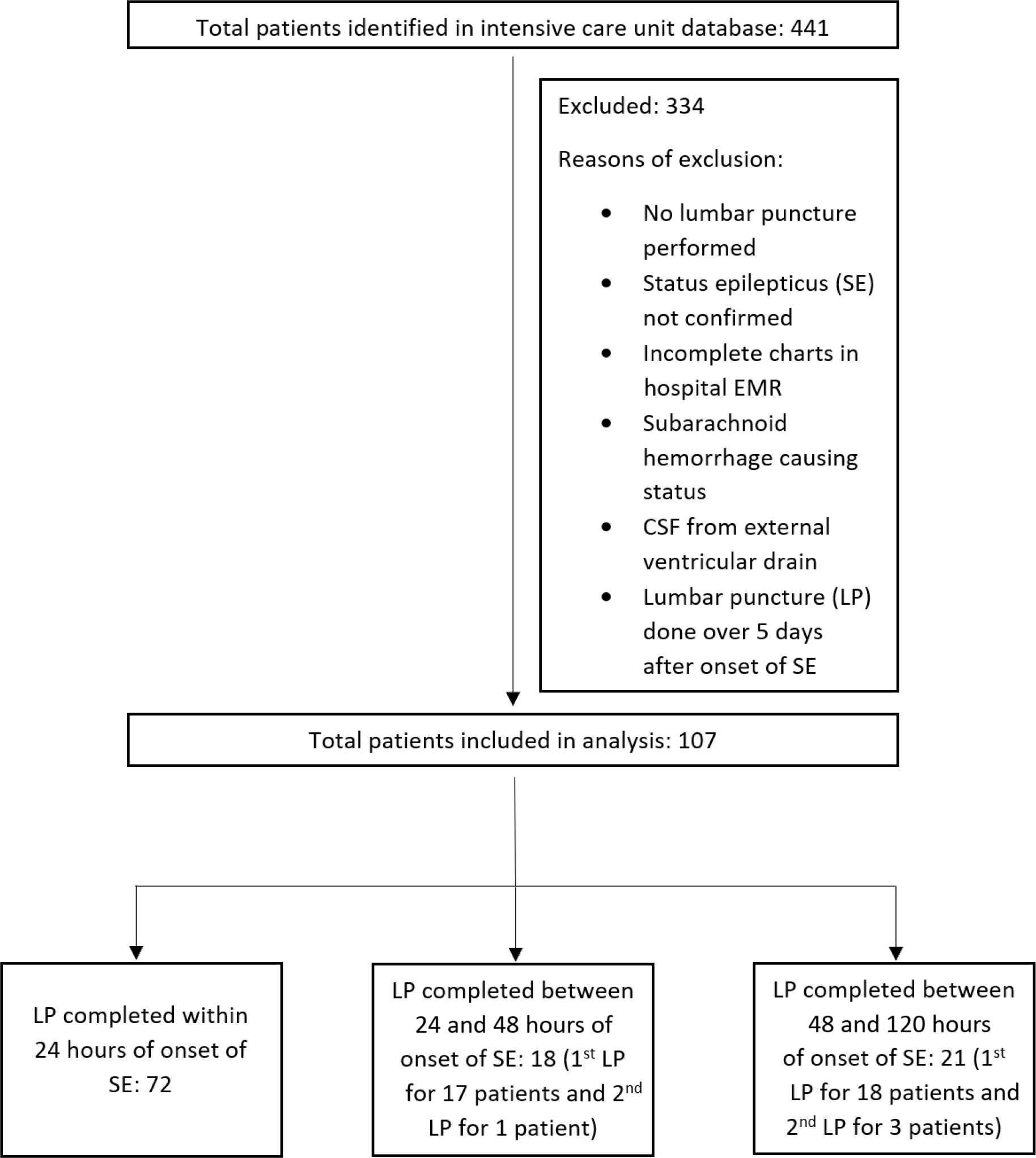
Figure 1: Consolidated Standards of Reporting Trials – style diagram depicting exclusion criteria and different categories of patient charts (N = 107 patients, 4 patients had two separate LPs hence LP N = 111).
Data Collection and Analysis
We collected the following CSF parameters including WBCs, protein, glucose, lactate, lactate dehydrogenase, gram stain, culture, and presence of autoantibodies. We also collected quantities of serum/whole blood glucose, serum albumin, serum WBC count within 8 hours from the time of LP. All these variables were handled as continuous variables except for gram stain, culture, and presence of autoantibodies, which were considered nominal categorical variables.
Information on patient’s history of SE including type of SE (focal vs generalized, convulsive vs non-convulsive, refractory vs super-refractory), time between SE onset to LP, electroencephalography confirmation of SE, computerized tomography (CT) of brain, and magnetic resonance imaging (MRI) of brain was extracted from electronic medical records. These variables were treated as nominal categorical variables. For all subjects, time to first LP and CSF parameters were retrieved. However, in four patients who had a 2nd LP, the second CSF data were also obtained.
In this study, CSF pleocytosis was defined as a CSF WBC count more than 5 × 106/L. Abnormal CSF protein was defined as greater than 450 mg/L. Elevated CSF lactate content was defined as ≥2.4 mmol/L. In addition, CSF-serum glucose quotient (QGlu) was calculated by finding the ratio of CSF glucose to serum or whole blood glucose and was considered pathologic if < 0.5. Serum or whole blood glucose was used based on the measurement that was closest to the time of LP. Presence of peripheral pleocytosis was defined as > 11 × 103 WBC cells/µL in the serum. SE was determined to be super-refractory when it persisted beyond 24 hours despite effective treatment.
Assessment of the Effect of Time on CSF Pleocytosis
As SE-related pleocytosis due to neuroinflammation is time dependent peaking at 24 hours after onset, Reference Dinkel, MacPherson and Sapolsky18 we categorized our patients according to the time gap between onset of SE and LP into three groups: under 24 hours, between 24 and 48 hours, and between 48 and 120 hours.
Statistical Analysis
The primary objective of this study is descriptive as we are assessing whether in our sample of 107 patients, there are cases where SE per se leads to pleocytosis. This is achieved by ruling out alternate causes of increased WBC in all cases of CSF pleocytosis.
For our secondary and tertiary objectives, we used simple t-tests to determine differences between pleocytosis and non-pleocytosis groups, refractory and super-refractory groups, convulsive and non-convulsive groups, and focal and generalized SE groups. We also performed Pearson correlation to test if there exist any relationships between various CSF parameters, clinical outcomes, and serum values. A p-value < 0.05 was considered significant. Data are expressed either as median (quartile 1 - quartile 3), mean ± standard error or count (%).
Results
A total of 443 patients admitted to VGH ICU were given the diagnosis of SE during the study period. Of these, 107 met the study criteria and were included in the analysis. Of these, 72 had their LP completed within 24 hours of onset of SE (Figure 1). In total, 111 LPs were performed, with 4 patients receiving a 2nd LP. Demographics are presented in Table 1.
Table 1: Clinical characteristics of patients stratified by pleocytosis versus non-pleocytosis
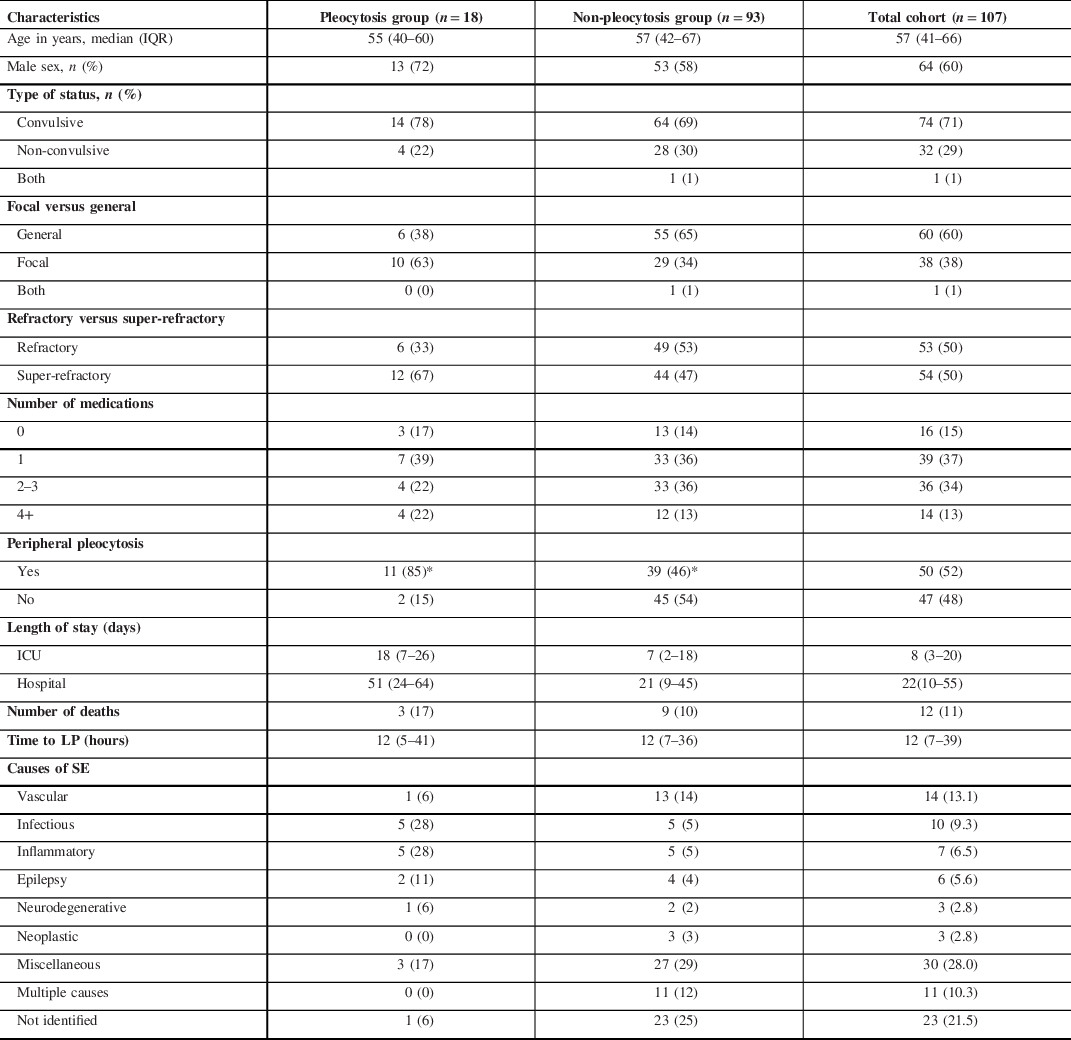
Values are expressed as either count (%) or median (Q1–Q3).*Significant difference p < 0.05. Peripheral pleocytosis is defined as > 11 × 103 WBC cells/µL of serum. Refractory SE is defined as SE that is refractory for up to 24 hours. Super-refractory SE is defined as SE that is refractory for over 24 hours. Missing data: n = 8 for focal versus general; n = 18 for number of medications; n = 14 for peripheral pleocytosis; n = 3 for length of stay in hospital, and n = 18 for length of stay in ICU.
CSF Pleocytosis
Of the 72 patients who had an LP within 24 hours of onset of SE, 12 patients had pleocytosis. All of these 12 patients had an etiology for their pleocytosis other than SE including infectious, autoimmune, iatrogenic, and vascular causes (Table 2).
Table 2: Causes, number of cases, CSF WBC count, and cause determined by for patients showing CSF pleocytosis within 24 hours post-SE
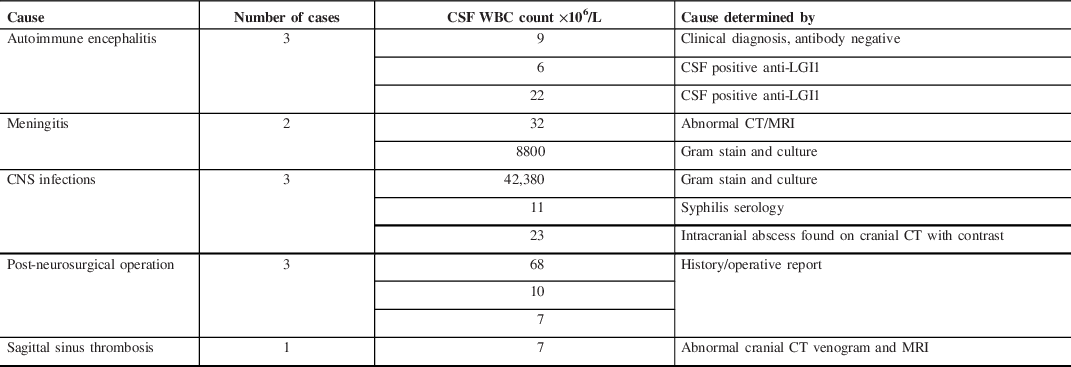
WBC=white blood cell counts; CSF=cerebrospinal fluid; anti-LGI1=antibodies to leucine-rich glioma inactivated 1; CT=computed tomography; MRI=magnetic resonance imaging.
Values are expressed as count.
In 18 patients who had their LP done between 24 and 48 hours after onset of SE, only 2 had pleocytosis. Both of these patients were not found to have any causes that could explain CSF pleocytosis suggesting that SE was most likely the sole cause of this phenomenon.
In 21 patients who had their LP done between 48 and 120 hours after onset of SE, 4 had pleocytosis. Causes of CSF pleocytosis in two out of four included autoimmune encephalitis. However, two patients were not found to have a cause for CSF pleocytosis besides SE. Additionally, out of three patients who had a repeat LP during this period, two showed CSF pleocytosis despite displaying no pleocytosis in their CSF sample taken in the first 24 hours post-SE.
Pleocytosis versus Non-Pleocytosis Groups
No significant difference was found in CSF protein and CSF glucose between pleocytosis and non-pleocytosis groups, despite accounting for different time categories. Even in patients with no CSF pleocytosis, 51% (44/87) showed elevated CSF protein, compared to 63% (10/16) of patients in the CSF pleocytosis group (p = 0.38). Similarly, CSF glucose was found to be elevated in 56% (49/87) of patients in the non-pleocytosis group compared to 60% (9/15) in the pleocytosis group (p = 0.79).
We found that in the pleocytosis group, there was a significantly higher serum WBC count (14.7 × 109/L vs 11.1 × 109/L; p = 0.026). In addition, 85% of the pleocytosis group displayed pleocytosis in the serum as compared to 46% of the non-pleocytosis group (p = 0.010). Among those with peripheral pleocytosis, 25% (16/64) of patients had CSF pleocytosis as compared to 4.3% (2/47) of patients with no peripheral pleocytosis. No other significant differences were found between pleocytosis and non-pleocytosis groups in Qglc, CSF lactate, serum albumin, serum glucose, and in any outcome variables including number of antiepileptic medications, as well as length of hospital and ICU stay. There was no significant difference in the proportion of super-refractory and refractory SE between pleocytosis and non-pleocytosis groups (Table 3).
Table 3: Laboratory and clinical findings in pleocytosis and non-pleocytosis groups and in the entire cohort

Values are expressed as either median (Q1–Q3) or count (%). WBC=white blood cell counts; CSF=cerebrospinal fluid; Qglc=CSF/serum or whole blood glucose quotient. WBC path=WBC > 5 × 106/L; protein path: protein > 450 mg/L; lactate path=lactate > 2.4 mmol/L; Qglc path=Qglc < 0.5. p Values denote difference between pleocytosis and non-pleocytosis groups; *n = 3; **n = 20; ***n = 23. Missing data: n = 8 for CSF protein; n = 88 for CSF lactate; n = 32 for Qglc; n = 7 for CSF glucose; n = 14 for serum WBC.
Effects of Type of SE on CSF/Serum Parameters and Clinical Outcomes
When comparing refractory versus super-refractory SE, CSF lactate was significantly higher in the refractory group than the super-refractory group (3.9 vs 2.6; p = 0.004). The super-refractory group was found to have a significantly higher number of medications at discharge, as well as longer stay in ICU and in hospital (Table 4). In addition, the super-refractory SE patients demonstrated a significantly higher rate of mortality during hospital stay with 11 out of 54 patients passing away (20.4%), as compared to 1 out of 53 patients (1.9%) passing away in the refractory SE group (p = 0.0025).
Table 4: Lab values and clinical outcomes in super-refractory and refractory SE patients and the corresponding p values
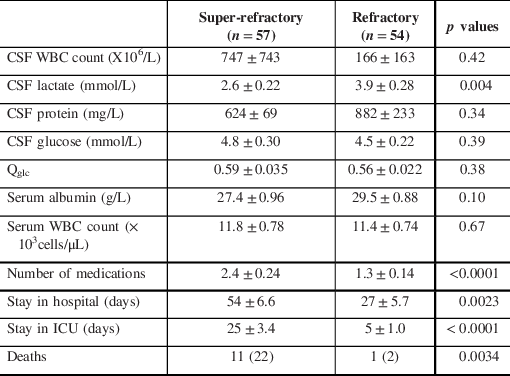
Values are expressed as mean ± standard error or count (%). WBC=white blood cell counts; CSF=cerebrospinal fluid; Qglc=CSF/serum or whole blood glucose quotient. Refractory SE is defined as SE that is refractory for up to 24 hours. Super-refractory SE is defined as SE that is refractory for over 24 hours.
Those with non-convulsive SE were found to have a significantly longer stay in ICU (24 ± 29 days vs 12 ± 13 days; p = 0.043) and a significantly longer stay in hospital (67 ± 66 days vs 30 ± 35 days; p = 0.0084) compared to convulsive SE.
Similarly, we found that those with focal SE had a significantly longer duration of stay in hospital (58 ± 58 days vs 32 ± 41 days; p = 0.031) compared to generalized SE. No other relationships between clinical SE characteristics, and CSF parameters, serum laboratory values, and clinical outcomes were found.
Correlation Analysis
We found a positive correlation between the number of antiseizure medications and duration of stay in ICU (r = 0.35; p = 0.0016) and hospital stay (r = 0.33; p = 0.0012), validating the use of all three as outcome variables. There was also a positive correlation between length of hospital stay and duration of ICU stay (r = 0.67; p < 0.0001).
Variables that displayed an association with outcome variables include CSF WBC count and Qglc. CSF WBC was positively correlated with length of hospital stay (WBC: r = 0.44; 95% CI: 0.26–0.59; p < 0.0001), whereas Qglc was found to be negatively associated with duration of hospital stay (r = −0.31; 95% CI: −0.51 to −0.072; p = 0.011).
CSF WBC was also positively correlated with CSF protein (r = 0.31; 95% CI: 0.13–0.48; p = 0.0014). Additionally, CSF WBC count was found to be negatively correlated with Qglc (r = −0.37; 95% CI: −0.55 to −0.16; p = 0.0008) and CSF glucose (r = −0.28; 95% CI: −0.45 to −0.090; p = 0.0042).
We also found that time to LP was negatively correlated with CSF lactate (r = 0.042; 95% CI: −0.71 to −0.017; p = 0.0424).
Discussion
The primary purpose of this study was to assess whether SE per se could lead to CSF pleocytosis. Our results indicate that in both refractory and super-refractory SE, when the LP was performed in less than 24 hours from the onset of SE, CSF pleocytosis was only noted in the setting of a secondary cause of pleocytosis other than SE. The secondary causes included CNS infection, autoimmune encephalitis, recent neurosurgical operation, and superior sagittal sinus thrombosis, which are known to cause CSF pleocytosis independent of SE. Reference Egelund, Ertner, Kristensen, Jensen, Benfield and Brandt7,Reference Wang, Sun and Liu21,Reference Alvis Miranda, Castellar-Leones, Elzain and Moscote-Salazar22,Reference Lee and Lee23 In our study, the refractory SE-induced pleocytosis was rare and only noted beyond 24 hours, which can be explained by delayed neuroinflammation process in the setting of SE. Reference Manley, Bertrand, Kinney, Hing and Sapolsky12,Reference Dinkel, MacPherson and Sapolsky18
To the best of our knowledge, this is the first adult study with a large sample size to indicate that refractory and super-refractory SE per se did not cause CSF pleocytosis when the sample was obtained in the first 24 hours of SE onset. This suggests that CSF pleocytosis found within the first 24 hours should be extensively investigated as it likely indicates an underlying pathology. Our finding contradicts several studies performed more than two decades ago, which demonstrated that SE in the absence of secondary causes may lead to CSF pleocytosis. Reference Prokesch, Rimland, Petrini and Fein2,Reference Aminoff and Simon4,Reference Schmidley and Simon8,Reference Barry and Hauser3,Reference Edwards, Schmidley and Simon24 In those studies, even after excluding known causes of CSF pleocytosis, up to 20% of adult patients had a CSF with increased WBC due to the seizures or SE per se. Reference Prokesch, Rimland, Petrini and Fein2,Reference Schmidley and Simon8,Reference Edwards, Schmidley and Simon24 However, those older studies were either conducted with no brain imagining or they used less sensitive techniques such as CT scan as opposed to MRI of brain Reference Prokesch, Rimland, Petrini and Fein2,Reference Aminoff and Simon4,Reference Schmidley and Simon8,Reference Barry and Hauser3,Reference Edwards, Schmidley and Simon24 and may lack some of the new developments in laboratory techniques such as polymerase chain reaction and autoantibody assays. Compared to the older studies, we have taken advantage of most up-to-date technology in brain imaging, immunology, and molecular biology to rule out secondary causes of SE. In addition, we have specifically looked at CSF pleocytosis in the first 24 hours and beyond 24 hours after the onset of SE to begin to understand the effect of time on pleocytosis post-SE.
A few recent studies have reassessed CSF findings in seizures, infectious SE, and non-infectious SE. The findings of these studies indicate that postictal CSF pleocytosis is rarely caused by SE per se. Reference Malter, Choi and Fink5,Reference Tumani, Jobs, Brettschneider, Hoppner, Kerling and Fauser6,Reference Scramstad and Jackson25 Our study is different as these were broader studies that even included the non-epileptic seizures and seizures without SE, Reference Tumani, Jobs, Brettschneider, Hoppner, Kerling and Fauser6,Reference Scramstad and Jackson25 and therefore their sample size specific to SE is small. In addition, some of these studies did not rule out all secondary causes of SE including the autoimmune etiologies and may not have taken into account the effect of time on CSF pleocytosis. Reference Malter, Choi and Fink5,Reference Tumani, Jobs, Brettschneider, Hoppner, Kerling and Fauser6,Reference Scramstad and Jackson25
Pleocytosis and Non-Pleocytosis Group Comparison
In terms of differences between patients who displayed CSF pleocytosis in any time category versus those who did not demonstrate any CSF pleocytosis, the only significant difference is serum WBC count. The pleocytosis group had a higher proportion of peripheral pleocytosis. This suggests that the CSF pleocytosis might be caused by the movement of WBC from serum to CSF due to breakdown of BBB. This finding is in agreement with the results of a previous pediatric study that also suggested that peripheral pleocytosis is associated with CSF pleocytosis. Reference Johnson, Michelson and Lyons26
The CSF protein and glucose were not significantly different between pleocytosis and non-pleocytosis groups.
Effect of Refractory SE on Other CSF Parameters
CSF Protein
Majority of the samples displayed elevated CSF protein suggesting that CSF protein elevation was likely present regardless of pleocytosis and underlying insidious pathology. This is similar to a previous study, where elevation in CSF protein was seen in 43% of patients with SE. Reference Hariharan, McBride, Cherikh, Tolleris, Bresnahan and Johnson27 In previous studies, CSF protein has been correlated with age, male sex, generalized seizures, and refractory SE. However, in our cohort of refractory SE, the only significant positive correlation of CSF protein was found with CSF WBC count. Reference Malter, Choi and Fink5,Reference Chatzikonstantinou, Ebert and Hennerici28 Elevated CSF protein was not associated with any of the three outcome variables either. In the absence of secondary cause for SE, the elevated CSF protein in our sample was most likely due to the SE per se. Therefore, we conclude that an isolated mildly to moderately elevated CSF protein may not mandate further workup and intervention.
Qglc
In our study, Qglc was inversely correlated with CSF WBC and a longer hospital stay. This is in contrast with previous studies that did not find any clinical relevance to Qglc. Reference Malter, Choi and Fink5,Reference Chatzikonstantinou, Ebert and Hennerici28 Our study found Qglc as a tool to predict the presence of CSF infection or other secondary causes and may have relevance in predicting clinical outcomes. However, future studies are required to prove its utility.
CSF Lactate
We found that there was a positive correlation between CSF glucose and CSF lactate. However, we did not find any correlation between either of our three outcome variables and CSF lactate, which is consistent with the findings by Malter et al. Reference Malter, Choi and Fink5 However, our results are in contrast with previous studies that demonstrated a link between elevated CSF lactate and morbidity and mortality as it has been postulated to be caused by excessive metabolic brain activity. Reference Tumani, Jobs, Brettschneider, Hoppner, Kerling and Fauser6,Reference Calabrese, Gruemer, James, Hranowsky and DeLorenzo19
Effects of Type of SE on CSF/Serum Parameters and Clinical Outcomes
Non-convulsive, focal, and super-refractory characteristics were associated with worse clinical outcomes including more medications on discharge, longer hospital stay, and/or longer ICU stay.
Study Limitations
The main limitation of our study is related to the retrospective design, which increases the chance of selection bias and information bias. To minimize the bias, we have selected our patients based on a clear inclusion and exclusion criteria, and every eligible subject was included. Therefore, our selection bias will not be a major concern for internal validity; however, our results may not be generalizable to other groups and populations. Additionally, due to the observational nature of this study, we were unable to account for confounding variables that may have led to these results.
In this study, we are also unable to confirm whether the cases with pleocytosis within the first 24 hours of SE are solely related to the underlying cause or whether SE-related pleocytosis is also a contributory factor. However, given that CSF pleocytosis within the first 24 hours of SE was only seen when there was an underlying etiology, it may indirectly indicate that CSF pleocytosis was related to the underlying etiology.
Another limitation of this study is that subgroups based on time of CSF collection have small sample size. This is seen in CSF samples collected between 24 and 48 hours, and those collected after 48 hours as these subgroups only include 18 and 21 patients, respectively. Therefore, the data in these subgroups should be interpreted with reservation and the results for these groups may not be generalizable. However, this limitation is mostly related to rareness of this condition. In addition, we were unable to measure Qalb due to lack of availability of CSF albumin in our charts. As a result, we are unable to comment on BBB breakdown in our patient population.
Conclusion
While there is a possibility of CSF pleocytosis following SE, the likelihood of seeing this phenomenon, as a result of refractory SE or even super-refractory SE per se, is very low within the first 24 hours of seizure onset. This is in part due to the time it takes for SE-related neuroinflammation to ensue as it peaks at 24 hours after onset of seizures. Therefore, we suggest that any pleocytosis noticed within the first 24 hours of the onset of refractory SE should not be attributed solely to SE. Instead, one should perform extensive investigation and potentially initiate empirical antimicrobial or immunomodulation therapy based on clinical suspicion to address the underlying cause of SE before it leads to further non-reversible neurological injury.
Acknowledgment
This study is partially financially supported by funding from Division of Neurology, UBC Department of Medicine.
Disclosures
None.
Statement of Authorship
S.B. contributed to methodology, ethics application, data collection, data analysis, and manuscript writing. D.G. contributed to methodology and manuscript review for quality assurance. Y.A.K. contributed to methodology and manuscript review for quality assurance. F.M.A. contributed to methodology, ethics application, data analysis, results review, and manuscript review.









