Stroke has emerged as the second commonest cause of mortality worldwide. 1 In Mexico, ischemic stroke represents the fourth cause of death and is an important health care issue. 2 Moreover, ischemic stroke in young adults aged between 15 and 45 years old has a prevalence ranging from 3 to 5%.Reference Ferro, Massaro and Mas 3 , Reference Putaala, Metso and Metso 4 In arterial thrombosis at young age, traditional risk factors such as gender, smoking, hypertension, diabetes mellitus, obesity and hypercholesterolemia do not fully explain the thrombotic risk. Therefore, additional factors must be present that contribute to the likelihood of developing arterial thrombosis, such as genetic factors.Reference Pruissen, Kappelle, Rosendaal and Algra 5
Previous studies have demonstrated that an impaired fibrinolytic system plays a major role in the pathogenesis of ischemic stroke.Reference Kristensen, Malm, Nilsson, Hultdin, Carlberg and Olsson 6 , Reference Kain, Young, Bamford, Bavington, Grant and Catto 7 Plasmin is the major enzyme in the fibrinolytic system and lyses fibrin clots. The conversion of plasminogen to plasmin is catalyzed by tissue plasminogen activator (tPA) and is inhibited by plasminogen activator inhibitor (PAI-1). Therefore, a high plasma level of PAI-1 is associated with reduced fibrinolytic activity and increased risk for thrombotic events.Reference Dawson and Henney 8 The promoter region of the PAI-1 gene contains a common single guanine nucleotide insertion/deletion polymorphism (4G/5G) situated 675 (base pairs) bp from the transcription start site Reference Dawson, Wiman, Hamsten, Green, Humphries and Henney 9 and it is associated with increased PAI-1 plasma levels. Several studies have demonstrated that PAI-1 levels represent a risk factor for ischemic stroke in some ethnic groups but the PAI-1 concentration was not influence by the 4G/5G polymorphism.Reference Xu, Li, Sheng and Liu 10 It has been demonstrated that 4G/5G polymorphism is significantly associated with an increased risk of stroke,Reference Bang, Park, Ahn, Shin and Hong 11 but other studies failed to confirm this association.Reference Ding, Nicklas, Fallin, de Rekeneire, Kritchevsky, Pahor, Rodondi, Li, Zmuda and Harris 12 - Reference Catto, Carter, Bamford, Davis and Grant 15 It has been described that this polymorphism represents a protector effect for ischemic stroke in young and older individuals,Reference Roest, van der Schouw and Banga 16 but results are still controversial.
One of the most important products of endothelial cells is nitric oxide (NO), a major mediator of endothelium dependent vasodilation made in the endothelial cells from l-arginine through the action of the homodimeric enzyme endothelial nitric oxide synthase (eNOS). Nitric oxide has critical roles in the regulation of vascular homeostasis and prevention of atherogenesis by inhibiting leukocyte, platelet activation and smooth muscle cell proliferation.Reference Lloyd-Jones and Bloch 17 There is a guanine to thymine (Glu298Asp) polymorphism at position 894 of the eNOS gene and the T allele (298Asp) had been described associated with an increased risk for coronary artery disease (CAD)Reference Cam, Sekuri and Tengiz 18 and hypertension,Reference Rossi, Taddei and Virdis 19 however, those findings have not been corroborated by others.Reference Lembo, De Luca and Battagli 20 Moreover, the Glu298Asp polymorphism has been identified as an independent risk factor for carotid atherosclerosis and ischemic stroke.Reference Zalba, Fortuño, San José, Moreno, Beloqui and Díez 21 - Reference Majumdar, Nagaraja, Karthik and Christopher 25
Those polymorphisms have not been analyzed in our Mexican population. Therefore, the aim of this study was to evaluate the effect of the Glu298Asp and 4G/5G polymorphisms on the risk for ischemic stroke in patients≤45 years of age.
MATERIALS AND METHODS
Study group
A total of 204 consecutive, unrelated patients ≤45 years old, with diagnosis of ischemic stroke were enrolled between January 2006 and June 2010. Diagnosis of ischemic stroke was considered in all patients after an acute focal neurological deficit with duration greater than 24 hours, confirmed by means of brain computed tomography or magnetic resonance imagery. Also, cardiac/transcardiac and carotid or vertebral artery sources of emboli as well as cervical arterial dissection were always excluded by means of transesophageal and transthoracic echocardiogram, cardiac magnetic resonance, magnetic resonance angiography and carotid and vertebral Doppler ultrasound. Patients with cerebral hemorrhage were not included in the study. We performed chromogenic assays to evaluate functional levels of antithrombin, protein C and protein S (Diagnostica Stago). We also determined lupus anticoagulant (DVV test, DVV confirm®; American Diagnostic, USA), modified activated protein C resistance (normal ratio >2.0, Coatest+APC Resistance V-S; Chromogenix, Sweden) and anticardiolipin antibodies (normal value<10 U; Sanofi Diagnostics Pasteur, France). Lupus anticoagulant and anticardiolipin antibodies tests were performed. The control group was constituted by 204 individuals without history of cerebrovascular disease. Age, sex, use of oral contraceptives and history of thrombotic events or drug abuse were recorded.
Other risk factors for atherothrombotic disease were noted. Patients of 45 years old or younger were included to minimize the effect of long-term environmental influences on disease etiology. Hypertension was defined if a subject fulfilled the European Society Cardiology criteria, or they were already being treated with antihypertensive drugs. A family history of CAD was defined as CAD or sudden death in a first-degree male relative younger than 55 years of age or a female relative younger than 65 years of age. Patients were considered smokers if they were currently smoking or had ceased within the last 12 months. Subjects were considered to be dyslipidemia if they had a cholesterol level of 200 mg/dl, or if they were being already treated. Individuals were considered with diabetes if they has fasting glycemia >126 mg/dl, or if they were already diagnosed as diabetic. The 4G/5G polymorphism in the PAI-1 gene, and the Glu298Asp in the eNOS gene, were determined in all participants.
Ethics
The study protocol was reviewed and approved by the Human Ethical Committee, and Medical Research Council of the Instituto Mexicano Del Seguro Social and conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Informed written consent was obtained from all subjects before enrollment.
DNA extraction and Genotyping
Genomic DNA was extracted from whole blood samples using standard methods (QIAamp DNA Blood Mini Kit, Qiagen GmbH, Hilden Germany). For detection of the 1Glu298Asp polymorphism on the eNOS, we used primer pairs to amplify a part of the eNOS gene containing exon 7, by polymerase chain reaction (PCR) with the following flanking intronic primers: (sense) 5’CATGAGGCTCAGCCCCAGAAC-3’and (antisense) 5’ AGT CAA TCC CTT TGC RGC TCAC-3’, followed by Mbo I (New England Biolabs, Beverly Mass) restriction endonuclease digestion for eight hours at 37° C and resolution by electrophoresis on 3% agarose gel (BIO-RAD Laboratories Hercules, CA). WI). Amplifications were performed in a Thermo Cycler (Applied Byosistem, Foster CA). Polymerase chain reaction was performed by denaturation at 94°C for 30 seconds (sec), annealing segment at 60°C for 30 sec, and extension at 72°C for 30 sec, repeated for 30 cycles. The resulting 206 base pair (bp) polymerase chain reaction product was cleaved into two smaller fragments of 119 and 87 bp in the presence of a T nucleotide at 894 (corresponding to Asp 298) but not in its absence (wild type). Digestion fragments were resolved by electrophoresis on 2.5% agarose gel stained with ethidium bromide (Figure 1).

Figure 1 Genotypic determination of the polymorphism Glu298Asp of the eNOS gene. Agarose gel electrophoresis of PCR products after endonuclease restriction with enzyme MboI. Lane 1 represents undigested PCR amplification of 266 bp fragments. Line 2, Glu/Glu homozygote, line 3 Glu/Asp heterozygote, and lane 4, Asp/Asp homozygote. M represents 100 bp ladder. DNA fragment sizes, in base pairs, are indicated by numbers on the right.
Genotyping of 4G/5G polymorphism in the PAI-1 promoter region was performed by PCR using the following oligonucleotides: 5´CACAGAGAGAGTCTGGCCACGT-3´(sense) and 5´CCAACAGAGGACTCTTGGTCT-3´ (antisense). The reaction conditions were as follow: initial denaturation at 94°C for three minutes (min) followed by 30 cycles of denaturation at 94° C for three sec, alignment at 60°C for 30 sec, and an extension step at 72°C for 30 sec, followed by a final linear extention at 72°C for one min. Products were subjected to digestion with restriction enzyme BsI I (New England Biolabs, Beverly, Massachussetts, USA) at 55°C. The DNA fragments were separated by electrophoresis in 3% agarose gel (BIO-RAD Laboratories, Hercules, California, USA) and visualized using ethidium bromide. All samples were processed in duplicated. Some were subject to sequencing (Figure 2).

Figure 2 Agarose electrophoretic gel analysis of the 4G/5G polymorphic region of PAI-1. M represents 100bp molecular weight marker; lines 1 represents the 99-98 bp polymorphic fragment, line 2 the fragment corresponding to the genotype 4G/5G (98-77bp) after digestion with BsI I (98bp), line 3 represents the genotype 4G/5G after digestion with BsI I (98bp), line 4 the fragment corresponding the genotype 5G/5G (77 bp).
Statistical analysis
Continuous data are expressed as the mean ± standard deviation (SD); categorical data are expressed with percentages. The significance of differences between continuous variables was determined by Student’s t test. Differences between categorical variables, was determined with the chi square test. Adjusted odds ratio (OR) was calculated by multivariate logistic regression analyses for 4G/5G and Glu298Asp polymorphism and traditional cardiovascular risk factors. A P value <0.05 was considered as statistically significant. All statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) statistical software package (version 15: SPSS Inc, Chicago, IL, USA).
RESULTS
Baseline characteristics of patients and controls are shown in Table 1. Ethnic background of all patients and controls was Mexican. All patients were 45 years of age or less at the time of the ischemic stroke. The original sample was conformed by 251 patients. However, we found 33 patients with thrombophilic conditions and those patients were excluded from this study. Fourteen patients were diagnosed with cerebral hemorrhage and also were excluded. In the final sample of 204 patients, we found evidence of 153 patients with cryptogenic stroke and 51 patients with small-vessel disease. Mean age for the group of patients was 34.1±5.4 while mean age for the control group was 33.5±6.1 years. There was no difference in terms of gender between both groups (P=0.5), or diabetes mellitus between the groups (P=0.18), patients with stroke had a higher prevalence of conventional risk factor for atherothrombotic disease. We performed thrombophilia screening only in the patients. We found protein S deficiency (7), protein C deficiency (1), anticardiolipin antibodies (15), lupus anticoagulant (6), acquired activated protein C resistance (4) and antithrombin deficiency (1). One patient had two thrombophilic conditions. All 33 patients were excluded from this study.
Table 1 Demographic and clinical characteristics of patients with ischemic stroke and controls
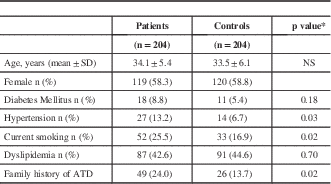
* t test for continuous variables; chi square or Fisher exact test for proportions
ATD=Atherothrombotic disease NS= Not significant
Hypertension was higher in the group of patients (13.2% vs. 6.7%, OR=2.07; 95% CI=1.01-4.31, P=0.03), as was smoking frequency (25.5% vs. 16.9%, OR=1.77; 95% CI=1.06-2.97, P=0.02). There was no significant difference between the groups in terms of dyslipidemia (P=0.7).
Table 2 shows the genotype distribution and allele frequency of Glu298Asp polymorphism among patients and controls and directly compares each genotype and allele among both groups. There was a significant difference in the genotype distribution between both groups (P=0.001). The genotype distribution in the control group was 146 individuals (71.6%) homozygous for the allele Glu, 52 (25.5%) heterozygous Glu/Asp, and 6 (2.9%) homozygous for the allele Asp. There was significant difference in the allele frequency (P=0.001). For patients and controls, frequencies of the Glu allele were 73.5% and 84.35%, respectively. The univariate analysis identified the Asp allele as a risk factor for ischemic stroke (homozygous Asp/Asp and heterozygous Glu/Asp carriers), as compared with homozygous for Glu/Glu allele (OR=2.28, 95% CI =1.48-3.51, P=0.01).
Table 2 Genotype distribution and allele frequency of Glu298Asp and 4G/5G polymorphisms among patients with ischemic stroke and controls

Data presented are number (%) of patients. *=χ 2 test
Table 2 shows the genotype distribution and allele frequency of 4G/5G polymorphism among patients and controls and compares genotype and alleles among both groups.. There was a significant difference in the genotype distribution between both groups (p=0.001). The genotype distribution in the control group was 16 individuals (7.8%) homozygous for the allele 4G, 87 (42.6%) heterozygous 4G/4G, and 101 (49.6%) homozygous for the allele 5G. There was no significant difference in the allele frequency (P=0.13). For patients and controls, frequencies of the 4G allele were 34.35% and 29.0%, respectively.
Table 3; According to the adjusted ORs, four independent risk factors were associated with stroke: Asp carriage (GluAsp+AspAsp) (OR=1.75, 95% CI95= 1.0-2.14, P=0.02); smoking (OR=2.42, 95% CI=1.0-3.40, P=0.01); hypertension (OR=2.54, 95CI%=1.08-4.32, P=0.03), and familial history of atherothrombotic disease (OR=1.79, 95CI% 1.02-2.57, P=0.04).
Table 3 Multiple logistic regression analysis using ischemic stroke as the dependent variable
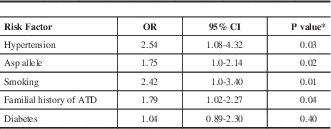
* chi square test multivariate logistic-regression; OR: adjusted odds ratio; ATD=Atherothrombotic disease
DISCUSSION
Ischemic stroke is the third leading cause of death worldwide and the most common cause of disability in the adult population. 1 Previous reports described the Glu298Asp and 4G/5G polymorphisms as risk factors for coronary and cerebral ischemic disease.Reference Lembo, De Luca and Battagli 20 , Reference Elbaz, Poirier, Moulin, Chédru, Cambien and Amarenco 22 - Reference Majumdar, Nagaraja, Karthik and Christopher 25 However, only a few studies were done in young patients and the results are controversial.Reference Isordia-Salas, Leaños-Miranda and Borrayo-Sánchez 26 Clinical and epidemiological studies have demonstrated an association between this polymorphism and the eNOS activity or endothelial function. In agreement with previous studies, we identified the Asp allele as an independent risk factor for ischemic stroke.Reference Elbaz, Poirier, Moulin, Chédru, Cambien and Amarenco 22 , Reference Szolnoki, Havasi and Bene 23 However, more important is the fact that this association persisted after adjustment for other classical risk factors for cerebral ischemic disease. Previously reports by Elbaz et al demonstrated, in a French population, that the Glu298Asp genotype was associated with brain infarction especially with lacunar stroke.Reference Elbaz, Poirier, Moulin, Chédru, Cambien and Amarenco 22 Further subgroup analysis demonstrated an association between lacunar strokes and low density lipoprotein (LDL) cholesterol level, with a possible synergistic action of lacunar stroke. The Glu allele frequency reported by Elbaz et al, was little lower compared with the frequency found in the present study: 60.7% vs 73.6%, respectively. The positive association between the polymorphism and ischemic stroke was corroborated by Cheng and co workers in Chinese population.Reference Cheng, Liu and Li 27 In a recent study, Szolnoki et al, demonstrated that the eNOS Glu298Asp or eNOS Asp298Asp genotypes combination with MTHFR 677TT or ACE DD increased the risk of ischemic stroke in a Caucasian population.Reference Szolnoki, Havasi and Bene 23 Several studies have demonstrated that the genetic variation Glu298Asp represents an independent risk factor for increased susceptibility to ischemic stroke.Reference Guldiken, Sipahi and Guldiken 24 , Reference Saidi, Mallat, Almawi and Mahjoub 28 In contrast, Markus and co worker showed no association between Glu298Asp and stroke or transient ischemic attack secondary to large vessel atherosclerosis, or with the degree of carotid stenosis in patients with cerebrovascular disease.Reference Zalba, Fortuño, San José, Moreno, Beloqui and Díez 21
Several experimental studies have demonstrated that NO inhibits many key steps of atherosclerotic disease and a defect in NO production could facilitate the progression of the atherosclerotic process. In fact, NO, besides its potent vasorelaxant effect, plays an important role in the regulation of blood pressure and regional blood flow. Also, NO is involved in the inhibition of platelet adhesion and aggregation, leukocyte adhesion and of smooth muscle cell migration and proliferation.Reference Arnal, Dinh-Xuan, Pueyo, Darblade and Rami 29 , Reference Radomski, Palmer and Moncad 30 The deficiency of NO is considered an important risk factor for endothelial dysfunction, which is an important contributor to ischemic stroke. Increased levels of homocysteine has been related to endothelial dysfunction. Recent studies demonstrated an association between the C677T polymorphism and hyperhomocysteinemia, with an increased risk for vascular occlusive disease. Earlier studies identified a common polymorphism (C677T) in the gene encoding the MTHFR as a genetic risk factor for stroke.Reference Croninn, Furie and Kelly 31 The results from the VITATOPS 32 were that daily administration of folic acid, vitamin B6, and vitamin B12 to patients with recent stroke or transient ischaemic attack was safe but did not seem to be more effective than placebo in reducing the incidence of major vascular events.
We have previously shown that the T allele of the MTHFR C677T is a risk factor for ischemic stroke in the same subgroup of Mexican subjects.Reference Isordia-Salas, Barinagarrementería-Aldatz and Leaños-Miranda 33 Arterial thrombus formation is believed to be an essential event in the pathogenesis of stroke. Therefore, it has been proposed that decreasing the fibrinolytic system plays on important role in this process.
In the present study, there was no association between the 4G allele and ischemic stroke in the same group of patients. Our results are similar to those described by de Sabino et al, in a Brazilian population with a negative association between the 4G allele and ischemic strokeReference de Paula Sabino, Ribeiro and Domingueti 34 . Also, similar results were reported by Jood et al, in 600 patients with acute ischemic stroke aged 18 to 69 years and 600 matched community controls.Reference Jood, Ladenvall and Tjärnlund-Wolf 14 Earlier results from Hoekstra et al, provided support for a protective effect of the 4G allele against stroke, which is notable given the direct relationship between stroke and PAI-1 activity.Reference Hoekstra, Geleijnse, Kluft, Giltay, Kok and Schouten 35 Jeppesen et al, demonstrated that 4G/5G polymorphism is associated with plasma levels but not with stroke risk in the elderly.Reference Jeppesen, Wilhelmsen and Nielsen 36 This finding is also consistent with two prospective studies showing reduced risk of stroke and cerebrovascular mortality for the 4G4G genotype in the elderly.Reference Roest, van der Schouw and Banga 16
The explanation of the reduced risk of stroke for PAI-1 4G/5G polymorphism remains speculative but may be found in a vascular pathological pathway that does not involve fibrinolysis.Reference Roest, van der Schouw and Banga 16 It has been demonstrated that PAI-1 serves a protective role, involving suppression of matrix-degrading enzymes in the plaque, resulting in limited plaque growth and prevention of abnormal matrix remodeling.Reference Shah, Falk and Badimon 37 Therefore, a local increase of tissue PAI-1 associated with the 4G allele may stabilize plaques, thereby reducing the risk of cerebrovascular disease. Another possible explanation may be that PAI-1 inhibits neurotoxicity of tissue plasminogen activation in the brain.Reference Roest, van der Schouw and Banga 16 , Reference Chen and Strickland 38
Our results are similar to those described for Babu et al,Reference Babu, Prabha and Kaul 39 in a Tunisian population. They found no association between the 4G/5G polymorphism and stroke. Moreover, Saidi et al,Reference Saidi, Slamia, Mahjoub, Ammou and Almawi 40 found a protective effect of 4G allele in an older population. They postulated a similar mechanism not related to fibrinolysis, possibly involving altered plaque stabilization, and /or through antagonism of tPA effects.
In a previous study by Fernandez et al, it was demonstrated in 165 patients, with ischemic stroke who received t-PA within three hours, that PAI-1 4G/5G patients had higher reoclussion rates compared with other genotypes (12.5% vs. 2.7% ). Also, the 4G/4G genotype was associated with poor functional outcome at the third month compared with the other genotypes.Reference Fernández-Cárdenas, Del Río-Espínola and Rubiera 41 However, that finding has to be confirmed with bigger samples in future studies.
Moreover, we had previously reported that the 4G/5G polymorphism is associated with an increased risk factor for ST segment elevation myocardial infarction in young Mexican individuals.Reference Isordia-Salas, Leaños-Miranda, Sainz, Reyes-Maldonado and Borrayo-Sánchez 42 Those results probably suggest that the 4/5G polymorphism may not have the same influence on ischemic stroke or is involved in different pathophysiological mechanisms in each of those territories.Reference Boncoraglio, Bodini, Brambilla, Carriero, Ciusani and Parati 43
Several studies had demonstrated that thrombosis disease is influenced by a complex interplay of procoagulant, anticoagulant, fibrinolytic, endothelial damage/dysfunction and inflammatory processes. Therefore, the present results suggest that in young patients with ischemic stroke, those polymorphisms may contribute to the premature endothelial dysfunction and thrombus formation.
In the present study, hypertension and smoking represented independent risk factors for stroke after adjustment for other variables.Reference Madonna, de Stefano and Coppola 44 Diabetes mellitus and dyslipidemia were not associated with ischemic stroke in this group of patients and these results are similar to previously described by others investigators. In contrast, studies in older populations with ischemic stroke found that diabetes mellitus and dyslipidemia were associated with this disease.Reference Pezzini, Grassi and Del Zotto 45 Based on these facts, we consider that thrombotic risk factors may not have the same influence on ischemic stroke in young patients as compared with older individuals and that the interaction between them and specific genetic variants may be different for each group of age.Reference Donnan, You, Thrift and McNeil 46 Therefore, a specific genotype-environmental combination may determine several possible phenotypes at different moments in life. Moreover, the specific influence of each known genetic abnormality associated with ischemic stroke may not be the same for every race. Therefore, it is desirable to establish the specific contribution of each genetic or environmental risk factor in individuals with different genetic backgrounds.Reference Vila 47 The knowledge of new genetic risk markers will permit recognition of a larger population at high risk of ischemic stroke, and this may lead to more effective prevention.Reference Gállego, Martínez Vila and Muñoz 48
Our study has several limitations, such as the small sample size, the lack of NO and PAI-1 level measurements and its correlation to genetic data. Meanwhile, the present study also has several strengths: 1) All ischemic stroke patients and controls has a similar ethnic background, and were matched by age and gender. 2) In the study patients of 45 years old or younger were included to minimize the effect of long-term environmental influences on disease etiology. 3) Laboratory genotyping was performed in a blinding manner, minimizing measurement bias.
In conclusion, our results demonstrated that the Asp allele from the Glu298Asp polymorphism represents an independent risk factor for ischemic stroke in young Mexican population. Moreover, hypertension, smoking and family history of thrombosis disease represented individual risk factors for ischemic stroke in our population. In contrast, the 4G allele was not associated with an increased risk for this disease in the same group of patients, as previously has been demonstrated in other populations.
Acknowledgments
This research was supported by El Fondo de Investigación en Salud IMSS grants (FIS/IMSS/PROT/G09/748); (FIS/IMSS/PROT/PRIO/13/023), CONACyT Consolidación de Grupos de Investigación modalidad repatriación (No. 050232) and Apoyo Complementario para Investigadores Nivel 1 No. (118254) and a Grant from Fundación IMSS, AC. (to I.I.S.).
Disclosures
None of the authors have anything to disclose.
JCEG and DS contributed equally to this study.
Conflicts of Interest
None declared.









