INTRODUCTION
Common source outbreaks of hepatitis A (HA) are fairly uncommon, but those occurring have been linked to eating raw or incompletely cooked contaminated foods, contaminated water or cooked food contaminated by infected food handlers [Reference Calder1–Reference Bloch7]. Hepatitis A virus (HAV) infection is preventable by vaccine and several formulations are available. In China, live-attenuated vaccine is manufactured by several companies and is widely used to prevent HA in schoolchildren.
In China, medical practitioners report both clinically suspected and IgM-confirmed HA through the national notifiable disease reporting system. Regulations also require the reporting of outbreaks. On 30 May 2006, township S in Sichuan Province, China, reported an outbreak of HA in students from three schools that served a town and 15 villages. Since the local health officials had, on 22–23 April 2006, vaccinated children aged from 1 to 18 years with liquid live-attenuated HA vaccine [LA-1 strain of HAV grown in human diploid cell (2BS) culture; Changchun Changsheng Life Sciences Ltd, China] [Reference Xu8], concern was raised that the vaccine had caused the outbreak. The local health unit requested assistance from Sichuan Centre for Disease Control and Prevention (CDC) in order to identify the possible source of infection and the mode of transmission and to recommend control measures.
METHOD
A case of HA was defined as onset of jaundice or anorexia since 1 April 2006 and a twofold elevation of alanine aminotransferase (ALT) and anti-HAV-IgM in a resident of or visitor to township S.
Several methods were utilized to search for HA cases: (1) Identification of students in the three schools and one kindergarten who at the routine morning check felt uncomfortable; any student who reported absent since 1 April was questioned. (2) Medical records were reviewed, in both township and county hospitals, for persons recently hospitalized with hepatitis. (3) The public were informed of the outbreak and parents asked to bring their children to the township hospital for a free examination if illness was suspected. (4) Village doctors were asked to report suspected hepatitis cases.
All patients were interviewed in person to obtain clinical data, and information about social gatherings, travel, employment, food consumption, sources of food and water, and HA vaccination.
A hypothesis that HA in students was acquired from snack foods sold from shops near the schools was tested through a case-control study. Ninety HA case-students were selected from the two most heavily affected schools and their exposures to snack foods and HA vaccine was compared with those of 107 asymptomatic control-students. Controls were selected at random from all asymptomatic students of the same schools excluding any student who reported having hepatitis previously. Exposures of cases and controls to snack foods sold near the schools were determined using a questionnaire to interview all cases and controls about eating snack foods, particularly those made from ice, during the probable exposure period of 27–30 April. Whether each case and control had received HA vaccine during the recent campaign was also determined. Exposure rates of cases and controls to each risk factor were compared using the cross product in Epi-Info version 3.3 (CDC, Atlanta, GA, USA) to estimate 95% confidence intervals (CI) of each odds ratio (OR). The Mantel–Haenszel test was used to calculate adjusted ORs and 95% CIs in multivariate analysis.
Water from a well – epidemiologically implicated as the source – was collected and tested to detect total and faecal coliform counts by the county CDC. The well-water samples and serum specimens from cases were sent to the Institute for Viral Disease Control and Prevention, China CDC, to detect HAV by reverse transcription–polymerase chain reaction (RT–PCR) [Reference Cao9–Reference Hutin12]. The nucleic acid of PCR products in the water sample and serum specimens from cases were sequenced [Reference Cao9]. The region of the VP1-P2A junction of the HAV genome was amplified. The length of external prime was 510 bp and the internal prime was 390 bp.
RESULTS
From 15 May 2006 to 28 June 2006, 116 HA case-patients were identified among residents of township S. They included 109 students, three kindergarten children and four adults. Symptoms in the 116 patients were typical for HA (Table 1). There were no deaths.
Table 1. Symptoms of hepatitis A in 116 cases, Sichuan, China, 2006
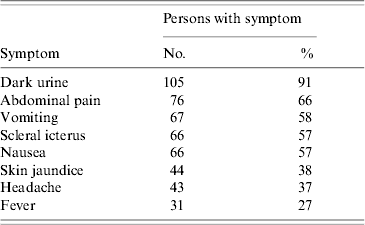
All 109 students, all three kindergarten children and three adult cases had onset of symptoms between 15 May and 14 June with peak incidence on 27 May suggesting a point-source exposure between 27 and 30 April (Fig. 1). The residences of 102 (88%) of 116 patients were distributed along one street in the main town where many shops sold snack foods to students. The other 14 students included seven who attended schools along the same street and another seven who attended primary schools in villages scattered throughout the township. The attack rate (AR) in the town primary school was highest, followed by town middle school, town kindergarten and town high school (Table 2). Age of HA cases ranged from 4 to 44 years (median age 9 years) and 89% were aged between 6 and 15 years. Fifty-two per cent (n=50) were male. In the three schools, the ARs for students who ate meals in the school cafeterias were lower than for those who ate outside the school, and the adjusted risk ratio (Mantel–Haenszel test) for eating at school was 0·48 (Taylor series 95% CI 0·28–0·84). The 75 cases from the primary school were distributed throughout all grades. In 22 classrooms, 21 had cases (with 1–7 cases in each). The distribution of cases did not cluster within classrooms (z=0·44, P>0·05). Six families had two cases; the other families with cases had one each.

Fig. 1. Epidemic curve for reported cases of hepatitis A by 3-day interval in township S, Sichuan Province, China, 2006.
Table 2. Attack rate by schools, grade, sex and eating at school cafeteria in hepatitis A outbreak, Sichuan, China, 2006

AR, Attack rate; RR, risk ratio; CI, confidence interval.
* Mantel–Haenszel adjusted RR and CI.
No common social event, restaurants, or community activities were identified during the possible exposure period of 27–30 April. A township water company served all three schools and households in the town. Some households owned private wells and used both the town running water and the well water.
Throughout the county, about 40 000 children were vaccinated by injection in April 2006, with a liquid formulation of live-attenuated HA vaccine. They included 2750 children from township S. The vaccine coverage rates were 57% in town primary school, 55% in town middle school and 39% in town high school. This was the only HA vaccination campaign undertaken in the previous 10 years. Indeed, the reason that the campaign took place in 2006 was that public health officials believed that recent low rates of HA suggested an accumulation of susceptible students and a potential for epidemics in 2006. Surveillance of hepatitis in the 47 townships of the county indicated that no other outbreak or cluster of HA had occurred.
Ninety case-students and 107 control-students were enrolled in the case-control study. The mean age of both cases and controls was 11 years and the male–female sex ratio for each was 1·1. Between 27 and 30 April, 34% of cases ate flavoured ice slush compared to 4·7% of controls (OR 11, 95% CI 4·0–29) and 51% of cases ate snow cones compared to 17% of controls (OR 5·2, 95% CI 2·7–10). No other exposures to snack foods sold around the schools were associated with HA. Sixty-two per cent of cases received HA vaccine on 22 and 23 April 2006 compared to 77% of control-students (OR 0·49, 95% CI 0·26–0·92). Multivariate analysis revealed a Mantel–Haenszel OR of 8·3 (95% CI 1·9–42) for snow cones and 4·1 for ice slush (95% CI 1·7–42) adjusted by vaccination status. HA vaccine showed no detectable protective effect (Mantel–Haenszel OR 0·63, 95% CI 0·31–1·3) (Table 3). Moreover, no detectable difference was found in serum ALT between unvaccinated (mean 802 IU/l, range 107–1481 IU/l) and vaccinated cases (mean 780 IU/l, range 115–1416 IU/l) (P>0·05 not significant, t test).
Table 3. Risks of hepatitis A by exposure in 90 cases and 107 controls, township S, Sichuan Province, China, 2006

OR, Odds ratio; CI, confidence interval; n.d., not determined.
In multivariate analysis, for vaccine, the OR=0·63 is adjusted for exposure to ice snacks by Mantel–Haenszel test. For ice slush and snow cone, they are compared to neither ice slush nor snow cones, the OR=4·1 for ice slush and OR=8·3 for snow cones are adjusted for vaccination by Mantel–Haenszel test.
Along the main street near the primary school, five shops sold snow cones and one shop (shop A) sold both ice slush and snow cones. Shop A also sold several other snacks made with ice or water and ran a public telephone bar. Of 75 cases and controls who reported eating ice slush or snow cones during the probable exposure days, 55 were cases and 20 were controls. Seventy-eight per cent (n=43) of the 55 cases who reported eating ice snacks reported that they bought them from shop A compared to 50% (n=10) of 20 control students who also ate ice snacks (OR 3·6, 95% CI 1·1–12). The shop-owners often made the ice for the snacks with water from their private well and sometimes from the town water system. The well located in the kitchen of shop A was 20 m deep and 30 cm wide; a plastic pipe served as the lining of the wall of the well. Water was pumped from the well to a storage tank. From the tank the water passed through a pipe to a three-way valve that was also connected to a pipe from the town water supply. From that valve either well or town water passed through >2 m of pipe to the tap where water was taken to make ice. Thus, the shop-owners could switch the tap between well water and town water by turning the valve. There was a toilet facility 3 m from the well. The sewage system in the town was lined with flagstones without mortar. Water from the well in shop A sampled on 23 June had >1600 faecal coliforms/100 ml. Tap water taken from the common pipe inside shop A had a total coliform count of 17 MPN/100 ml and faecal coliform count of 8 MPN/100 ml. Tap water from neighbouring shops had no detectable faecal or total coliforms/100 ml.
HAV RNA was detected by RT–PCR in water from the well in shop A. The nucleotide sequences of HAV (from 2934 bp to 3254 bp) that were obtained from the well-water sample and sera from 14 case-patients were identical. The outbreak sequence was most closely related (98·7%) to wild-type HAV strains DL3 recently isolated in China and deposited in Gene bank. It is not closely related (91·6%) to previously reported live-attenuated HAV vaccine strain H2 and LA-1 administered just before the outbreak. The outbreak sequence was genotype 1A and the live-attenuated HAV vaccine strain H2 and LA-1 were genotype 1B. It was unknown what type of HAV had circulated in this county prior to the outbreak, and only 10 HA cases had occurred throughout the county in the 3 months prior to the vaccination programme. Although a possible source case that might have contaminated the sewage with HAV was sought, one could not be found in the neighbourhood of the shop.
To prevent secondary spread as well as continuing infection from a common source, on 30 May county health officials administered human immune serum globulin (IG) to all asymptomatic students in this town (Fig. 1). After 7 days the number of new cases had fallen to almost zero. In addition, the students in the surrounding villages were given HA vaccine.
DISCUSSION
This investigation implicated ice slush or snow cones made by water from a polluted well in shop A as the vehicle for a HA outbreak. The elevated OR of 4·1 for ice slush and 8·3 for snow cones demonstrated their relationship with the outbreak. The environmental investigation of the well supported this conclusion and the nucleic acid sequence analysis of HAV isolated from the well-water sample and HAV patients provided final substantiation. It should be noted that the outbreak essentially ended one week after IG was given to all unaffected students. This prompt action possibly curtailed a more prolonged outbreak since the implicated well remained contaminated with HAV and was in use to the end of June.
Polluted water is a well known vehicle for HA. On the other hand, confirmation of well-water sources through identification of HAV from the water has been reported only twice before. Both these outbreaks indicated that HAV is stable in untreated well water [Reference Bloch7, Reference Gaston13]. HAV may persist for up to 6 months after an outbreak. Accordingly, the HAV genome detected in this well may easily have been present at the time of exposure of the cases.
Despite the suspicious time relationship between the vaccine and the outbreak, three findings dispel the idea that administration of HA vaccine caused this outbreak. First, the same lots of vaccine were administered in many other townships in April in the same county without subsequent HA outbreaks. Second, the case-control study showed vaccine was not a risk factor associated with illness. However, these data indicated that vaccination with live-attenuated vaccine around the same time of exposure had unacceptably low efficacy, although killed vaccine will prevent clinical hepatitis if given within 2 weeks following exposure [Reference Novak, Williams and Bell14]. Although the manufacturer of this vaccine had established quality control measures and passed quality control assessment and the vaccine was under strict temperature control at the county CDC, it is unknown if it was correctly handled during transport from the production factory to the county CDC. Third, the outbreak strain is genotype 1A but the live-attenuated HAV vaccine strain LA-1 is genotype 1B. Therefore the outbreak strain was different from the vaccine strain administered before the outbreak.
The main limitation of this investigation is that 38% (35/90) of case-students did not report exposure to ice snacks. Four possibilities might explain how they were exposed to HA. First, the focus was on the common snacks children had eaten during the exposure period. Shop A sold some other snacks made by contaminated water and may also have contributed to the case count. Second, some case-students could not remember whether they ate ice snacks during the possible exposure period. They could indeed have eaten some or shared ice snacks with their friends. Since the well remained contaminated after the estimated exposure period, they may have been exposed outside of this period. Third, some children were able to drink water from their own family's well or other shop wells which may also have been contaminated. Last, if the well water passed through a defect in the three-way valve into the town system, the children also could get HA by drinking piped, treated water. In addition, it was not possible to distinguish asymptomatic from uninfected control-students as interpretation of test results would have been confounded by the prior administration of vaccine or IG. Accordingly, the inclusion of asymptomatic and previously infected students in the control group could have occurred and would have biased the ORs towards 1·0.
This outbreak underscores the importance of a safe water supply for food preparation. Three possible methods of contamination of the ice include: (1) the shop-owner intentionally used well water to make one or more batches of ice, (2) the shop-owner, on one or more days, forgot to turn the valve to the town water supply before preparing a batch of ice, (3) residual well water in the pipe between the valve and the tap contaminated the ice after the valve was turned to town water. The design of the water system in this shop invited cross-contamination of polluted well water with the safe water system from the town. If the valve was faulty, water from the well could mix directly with the town water during periods of low pressure and contaminate water used by many shops and homes. Other shops, restaurants and homes had private wells close to the implicated shop. These may also have been contaminated with HAV or other pathogens. HAV and other enteric viruses have been identified in 32% of 448 groundwater sources in 35 states [Reference Abbaszadegan, LeChevallier and Gerba15]. Frequent contamination with these viruses has also been demonstrated in untreated water from municipal wells [Reference Borchardt, Haas and Hunt16]. Accordingly, raw well water should never be connected to the same pipes as the public water supply or to food preparation areas. In rural China, drinking from contaminated wells has also resulted in typhoid and dysentery outbreaks [Reference Yuan17, Reference Liu18]. This investigation highlights the potential for the transmission of HAV and other enteric diseases at retail food businesses that use water from unregulated private wells.
The provincial CDC advised the county officials to test all private wells inside the town for pollution and to prohibit the use of private well water for preparation of food for public sale. They also advised that residents should be prohibited from connecting private well water pipes to the public water system.
ACKNOWLEDGEMENTS
We thank the staff in Sichuan CDC for their contributions to this investigation.
DECLARATION OF INTEREST
None.








